NPs Basic Information
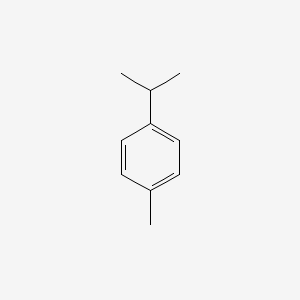
|
Name |
p-CYMENE
|
| Molecular Formula | C10H14 | |
| IUPAC Name* |
1-methyl-4-propan-2-ylbenzene
|
|
| SMILES |
CC1=CC=C(C=C1)C(C)C
|
|
| InChI |
InChI=1S/C10H14/c1-8(2)10-6-4-9(3)5-7-10/h4-8H,1-3H3
|
|
| InChIKey |
HFPZCAJZSCWRBC-UHFFFAOYSA-N
|
|
| Synonyms |
P-CYMENE; 4-Isopropyltoluene; 99-87-6; p-Isopropyltoluene; Dolcymene; Para-cymene; p-Cymol; Paracymene; 1-Isopropyl-4-methylbenzene; Camphogen; CYMENE; p-Methylcumene; 4-Cymene; Benzene, 1-methyl-4-(1-methylethyl)-; 2-p-Tolylpropane; Cymol; p-Methylisopropylbenzene; 1-Methyl-4-isopropylbenzene; Paracymol; p-Cimene; 4-Isopropyl-1-methylbenzene; 4-Methylisopropylbenzene; Cumene, p-methyl-; 1-Methyl-4-(1-methylethyl)benzene; p-methyl cumene; p-Isopropylmethylbenzene; 4-Methyl-1-isopropylbenzene; Cymene, p-; Benzene, 1-isopropyl-4-methyl-; Isopropyltoluene; 1-methyl-4-(propan-2-yl)benzene; 4-Isopropylbenzyl radical; FEMA No. 2356; para cymene; 1-methyl-4-propan-2-ylbenzene; 4-methyl isopropylbenzene; NSC 4162; 1-isopropyl-4-methyl-Benzene; Methyl-4-(1-methylethyl)benzene; 4-methyl-1-(propan-2-yl)benzene; p-Mentha-1,3,5-triene; 1G1C8T1N7Q; CHEBI:28768; NSC-4162; 1-Methyl-4-(1-methylethyl)-benzene; DSSTox_CID_6645; DSSTox_RID_78172; BENZENE,1-ISOPROPYL,4-METHYL P-CYMENE; DSSTox_GSID_26645; 4-Isopropyltoluol; CAS-99-87-6; 4-Cymol; HSDB 5128; EINECS 202-796-7; UNII-1G1C8T1N7Q; AI3-02272; p-methyl-Cumene; MML; 4-iso-Propyltoluene; MFCD00008893; PARACYMENE PF; p-Cymene, 99%; p-Cymene [UN2046] [Flammable liquid]; p-Cymene [UN2046] [Flammable liquid]; Carvacrol derivative, 8; P- Isopropylmethylbenzene; P-CYMENE [FHFI]; P-CYMENE [HSDB]; P-CYMENE [INCI]; CYMENE [MART.]; P-CYMENE [FCC]; P-CYMENE [MI]; 4-methyl isopropyl benzene; bmse000503; EC 202-796-7; Paramethyl-isopropyl-benzene; P-CYMENE [WHO-DD]; p-Cymene, analytical standard; 1-Methyl-4-isopropyl benzene; p-Cymene, >=97%, FG; CHEMBL442915; DTXSID3026645; NSC4162; BDBM248165; benzene, 1-methyl-4-methylethyl-; WLN: 1Y1 & R D1; ZINC968246; 1-(1-methylethyl)-4-methylbenzene; Tox21_201932; Tox21_300338; s5598; AKOS000121521; Benzene, 1-methyl-4(1-methylethyl)-; CCG-266123; LMPR0102090014; p-Isopropyltoluene, analytical standard; NCGC00247998-01; NCGC00247998-02; NCGC00254425-01; NCGC00259481-01; 4939-75-7; AC-34132; AS-11012; FT-0689324; S0664; EN300-21455; C06575; Q284072; W-100013; p-Cymene, certified reference material, TraceCERT(R); F8889-6466; Z104497772
|
|
| CAS | 99-87-6 | |
| PubChem CID | 7463 | |
| ChEMBL ID | CHEMBL442915 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.22 | ALogp: | 4.1 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.546 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.302 | MDCK Permeability: | 0.00001960 |
| Pgp-inhibitor: | 0.011 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.211 |
| 30% Bioavailability (F30%): | 0.931 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.728 | Plasma Protein Binding (PPB): | 94.38% |
| Volume Distribution (VD): | 2.139 | Fu: | 6.09% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.941 | CYP1A2-substrate: | 0.944 |
| CYP2C19-inhibitor: | 0.855 | CYP2C19-substrate: | 0.864 |
| CYP2C9-inhibitor: | 0.574 | CYP2C9-substrate: | 0.61 |
| CYP2D6-inhibitor: | 0.778 | CYP2D6-substrate: | 0.755 |
| CYP3A4-inhibitor: | 0.084 | CYP3A4-substrate: | 0.62 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.38 | Half-life (T1/2): | 0.276 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.037 |
| Drug-inuced Liver Injury (DILI): | 0.201 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.079 | Maximum Recommended Daily Dose: | 0.048 |
| Skin Sensitization: | 0.159 | Carcinogencity: | 0.386 |
| Eye Corrosion: | 0.958 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.03 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000026 | 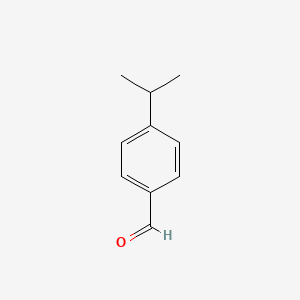 |
0.583 | D06GIP | 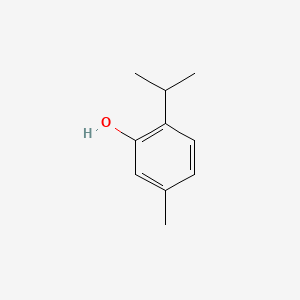 |
0.436 | ||
| ENC000368 | 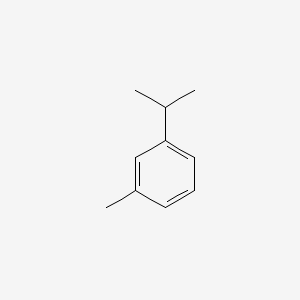 |
0.543 | D0R1QE | 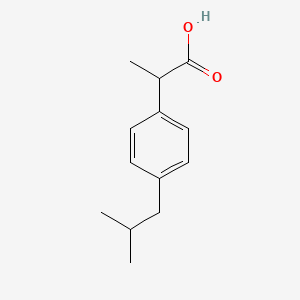 |
0.396 | ||
| ENC000233 | 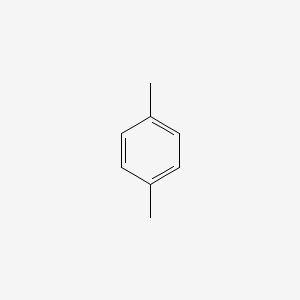 |
0.531 | D06YPU | 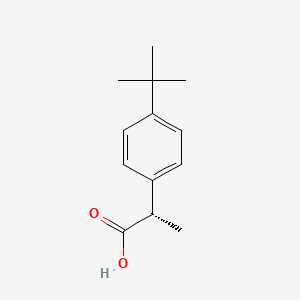 |
0.375 | ||
| ENC001121 | 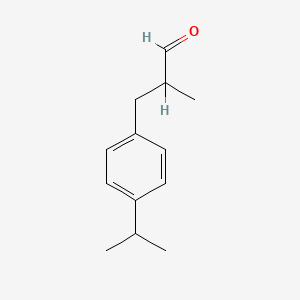 |
0.512 | D04VMT | 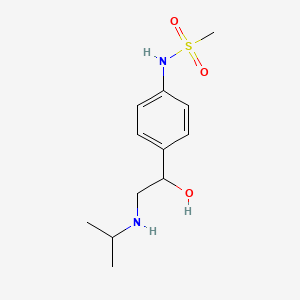 |
0.339 | ||
| ENC000796 | 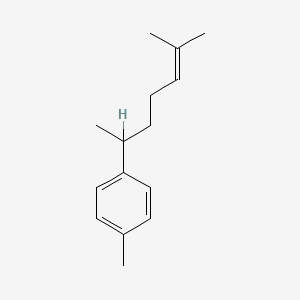 |
0.511 | D0X0WU | 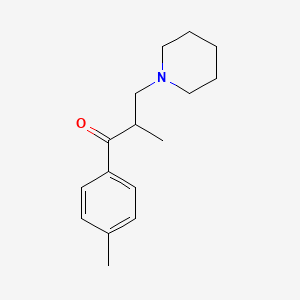 |
0.322 | ||
| ENC000221 | 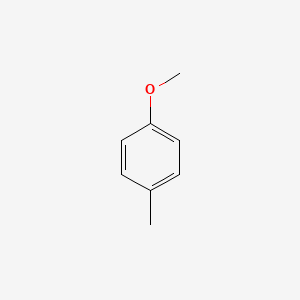 |
0.486 | D0YQ5L | 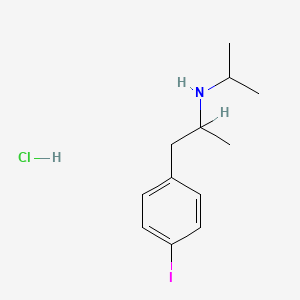 |
0.320 | ||
| ENC000086 | 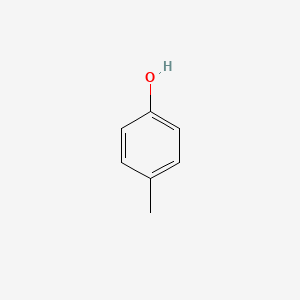 |
0.485 | D0A3HB | 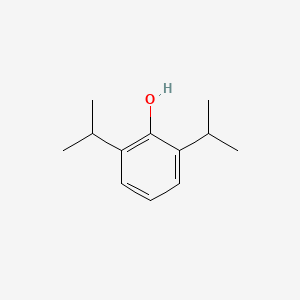 |
0.298 | ||
| ENC000347 | 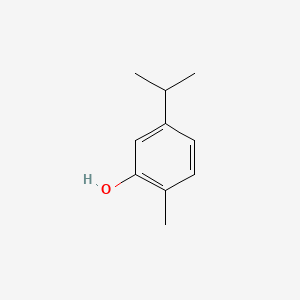 |
0.474 | D08GYO | 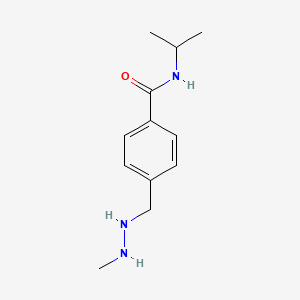 |
0.291 | ||
| ENC000098 | 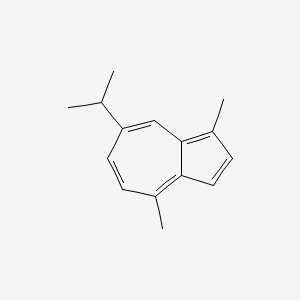 |
0.447 | D0W1RY | 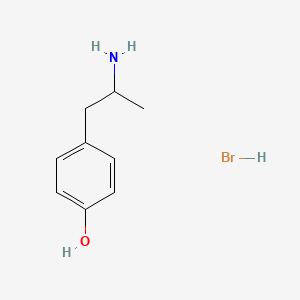 |
0.289 | ||
| ENC000191 | 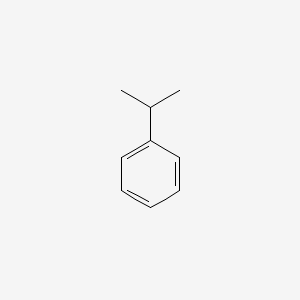 |
0.444 | D06OIV | 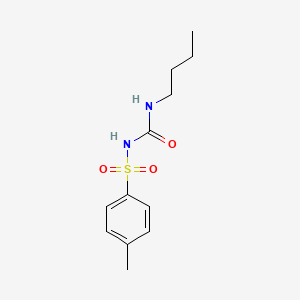 |
0.288 | ||