NPs Basic Information
|
Name |
Carvacrol
|
| Molecular Formula | C10H14O | |
| IUPAC Name* |
2-methyl-5-propan-2-ylphenol
|
|
| SMILES |
CC1=C(C=C(C=C1)C(C)C)O
|
|
| InChI |
InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)10(11)6-9/h4-7,11H,1-3H3
|
|
| InChIKey |
RECUKUPTGUEGMW-UHFFFAOYSA-N
|
|
| Synonyms |
CARVACROL; 5-Isopropyl-2-methylphenol; 499-75-2; Isopropyl-o-cresol; o-Thymol; Antioxine; Karvakrol; 2-p-Cymenol; 5-Isopropyl-o-cresol; Isothymol; 2-Hydroxy-p-cymene; Phenol, 2-methyl-5-(1-methylethyl)-; p-Cymen-2-ol; 2-Methyl-5-isopropylphenol; 5-Isopropyl-2-methyl-phenol; 2-Methyl-5-(1-methylethyl)phenol; 2-Methyl-5-(Propan-2-Yl)Phenol; 2-methyl-5-propan-2-ylphenol; 3-Isopropyl-6-methylphenol; p-Cymene, 2-hydroxy-; o-Cresol, 5-isopropyl-; 1-Hydroxy-2-methyl-5-isopropylbenzene; 6-Methyl-3-isopropylphenol; Phenol, 5-isopropyl-2-methyl-; CYMOPHENOL; FEMA No. 2245; Oxycymol; 1-Methyl-2-hydroxy-4-isopropylbenzene; Phenol, 3-isopropyl-6-methyl-; 2-Hydroxycymene; NSC 6188; CHEBI:3440; CHEMBL281202; 9B1J4V995Q; NSC-6188; 2-Methyl-5-(1-methylethyl)-Phenol; Cymene-2-ol, p-; Caswell No. 511; Cymenol; CCRIS 7450; HSDB 906; EINECS 207-889-6; EPA Pesticide Chemical Code 022104; BRN 1860514; UNII-9B1J4V995Q; AI3-03438; Hydroxy-p-cymene; Carvacrol Natural; Carvacrol,(S); p-Cymene-2-ol; Carvacrol, 98%; MFCD00002236; DENTOL; CARVACROL [FCC]; CARVACROL [MI]; CARVACROL [FHFI]; CARVACROL [HSDB]; CARVACROL [INCI]; CARVACROL [WHO-DD]; DSSTox_CID_22074; DSSTox_RID_79916; DSSTox_GSID_42074; SCHEMBL24734; 3-Isopropyl-6-methyl phenol; 3-Isopropyl-6-methyl-Phenol; 4-06-00-03331 (Beilstein Handbook Reference); BIDD:ER0492; Carvacrol, analytical standard; GTPL2497; Carvacrol, natural, 99%, FG; DTXSID6042074; Methyl-5-(1-methylethyl)phenol; p-Mentha-1,3,5-trien-2-ol; WLN: QR B1 EY1&1; FEMA 2245; NSC6188; Carvacrol, >=98%, FCC, FG; ZINC967563; HY-N0711; Tox21_301378; BDBM50240433; s3788; STL453136; AKOS000120828; AC-2688; CCG-266210; FS-4199; LMPR0102090017; MB00118; NCGC00256001-01; CAS-499-75-2; Isothymol (=2-Isopropyl-4-methyl phenol); Carvacrol Cymenol 5-Isopropyl-2-methylphenol; CS-0009729; FT-0627526; 2-HYDROXY-4-ISOPROPYL-1-METHYLBENZENE; EN300-21426; C09840; F17722; A827907; Q225543; Carvacrol, primary pharmaceutical reference standard; W-105999; BENZENE,2-HYDROXY,4-ISOPROPYL,1-METHYL CARVACROL; F8889-1978; Z104496566; BENZENE,2-HYDROXY,4-ISOPROPYL,1-METHYL CARVACROL
|
|
| CAS | 499-75-2 | |
| PubChem CID | 10364 | |
| ChEMBL ID | CHEMBL281202 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.22 | ALogp: | 3.1 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.648 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.436 | MDCK Permeability: | 0.00002350 |
| Pgp-inhibitor: | 0.007 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.932 |
| 30% Bioavailability (F30%): | 0.978 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.831 | Plasma Protein Binding (PPB): | 93.13% |
| Volume Distribution (VD): | 2.569 | Fu: | 8.52% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.936 | CYP1A2-substrate: | 0.953 |
| CYP2C19-inhibitor: | 0.753 | CYP2C19-substrate: | 0.749 |
| CYP2C9-inhibitor: | 0.517 | CYP2C9-substrate: | 0.877 |
| CYP2D6-inhibitor: | 0.851 | CYP2D6-substrate: | 0.878 |
| CYP3A4-inhibitor: | 0.329 | CYP3A4-substrate: | 0.437 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.335 | Half-life (T1/2): | 0.671 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.031 |
| Drug-inuced Liver Injury (DILI): | 0.085 | AMES Toxicity: | 0.034 |
| Rat Oral Acute Toxicity: | 0.217 | Maximum Recommended Daily Dose: | 0.513 |
| Skin Sensitization: | 0.298 | Carcinogencity: | 0.269 |
| Eye Corrosion: | 0.94 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.198 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
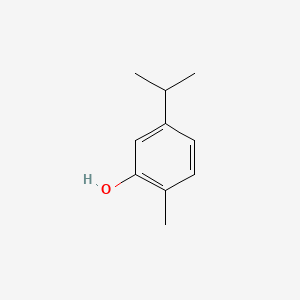
| ENC000804 | 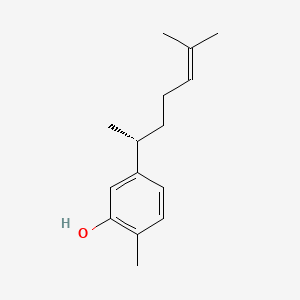 |
0.532 | D06GIP | 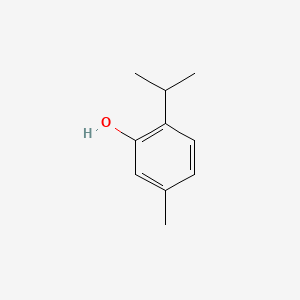 |
0.526 | ||
| ENC000368 | 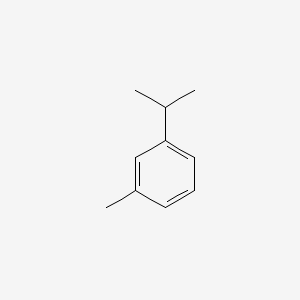 |
0.514 | D0I8FI | 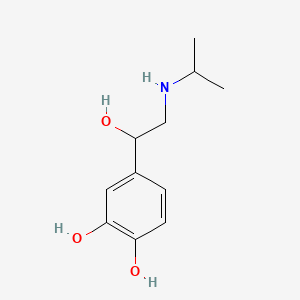 |
0.408 | ||
| ENC000098 | 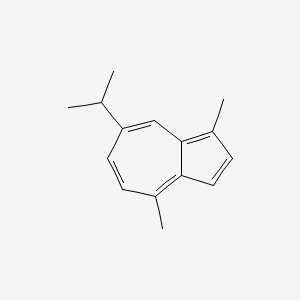 |
0.489 | D08HUC | 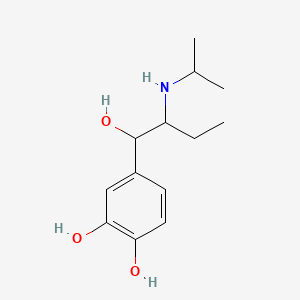 |
0.396 | ||
| ENC000199 | 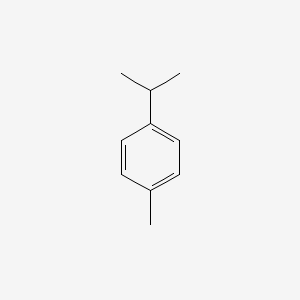 |
0.474 | D0A3HB | 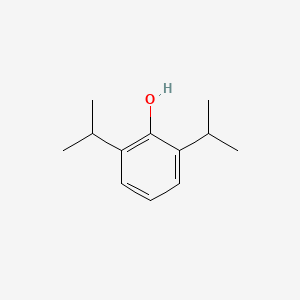 |
0.370 | ||
| ENC000365 | 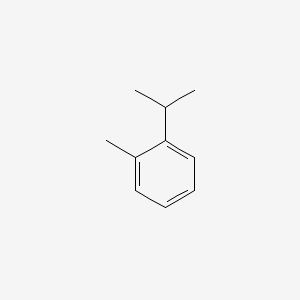 |
0.400 | D04PHC | 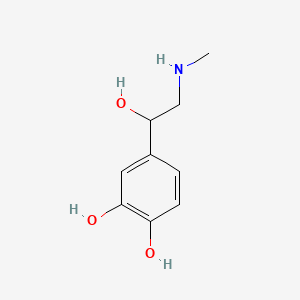 |
0.362 | ||
| ENC000180 | 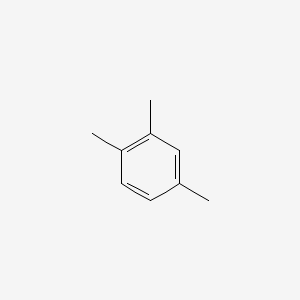 |
0.395 | D07MOX | 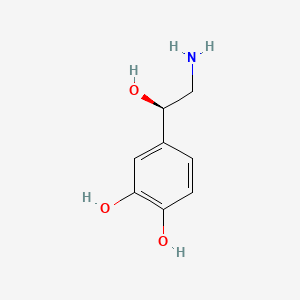 |
0.356 | ||
| ENC000471 | 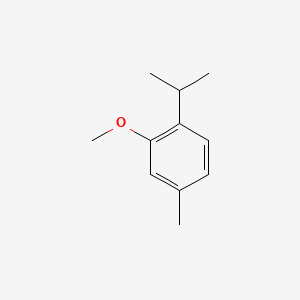 |
0.386 | D02ZJI | 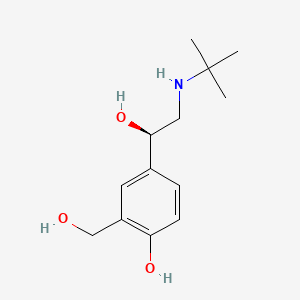 |
0.321 | ||
| ENC000338 | 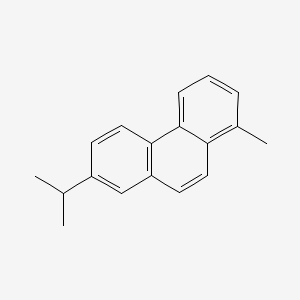 |
0.379 | D0K5CB | 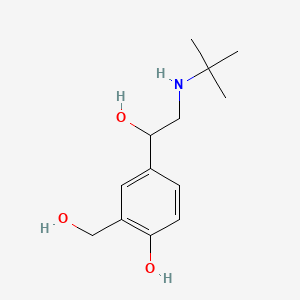 |
0.321 | ||
| ENC000028 | 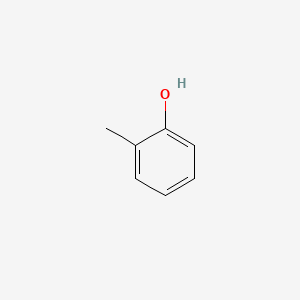 |
0.378 | D0I3RO | 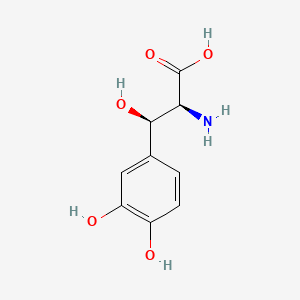 |
0.308 | ||
| ENC000026 | 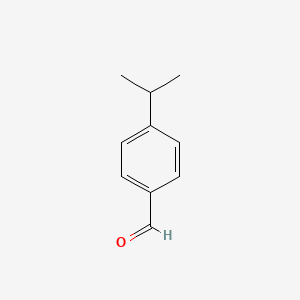 |
0.372 | D0EL2O | 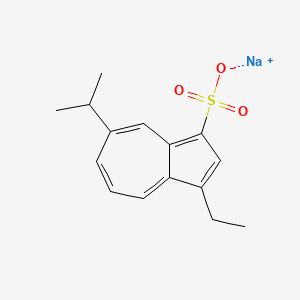 |
0.306 | ||