NPs Basic Information
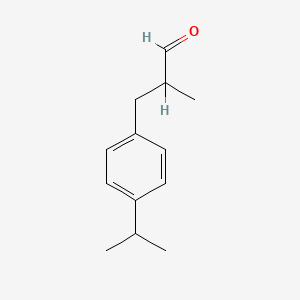
|
Name |
3-(4-Isopropylphenyl)-2-methylpropanal
|
| Molecular Formula | C13H18O | |
| IUPAC Name* |
2-methyl-3-(4-propan-2-ylphenyl)propanal
|
|
| SMILES |
CC(C)C1=CC=C(C=C1)CC(C)C=O
|
|
| InChI |
InChI=1S/C13H18O/c1-10(2)13-6-4-12(5-7-13)8-11(3)9-14/h4-7,9-11H,8H2,1-3H3
|
|
| InChIKey |
ZFNVDHOSLNRHNN-UHFFFAOYSA-N
|
|
| Synonyms |
103-95-7; Cyclamen aldehyde; 3-(4-Isopropylphenyl)-2-methylpropanal; 3-(4-Isopropylphenyl)isobutyraldehyde; Cyclamal; Cymal; Aldehyde B; 3-p-Cumenyl-2-methylpropionaldehyde; 2-Methyl-3-(p-isopropylphenyl)propionaldehyde; FEMA No. 2743; 2-methyl-3-(4-propan-2-ylphenyl)propanal; 3-(p-Isopropylphenyl)-2-methylpropionaldehyde; 2-Methyl-3-[4-(Propan-2-Yl)Phenyl]Propanal; Benzenepropanal, .alpha.-methyl-4-(1-methylethyl)-; xi-3-(4-Isopropylphenyl)-2-methylpropanal; 4U37UX0E1E; 3-(4-Isopropylphenyl)-2-methylpropionaldehyde; .alpha.-Methyl-p-isopropylhydrocinnamic aldehyde; Cyclaviol; Isopropylmethylhydrocinnamaldehyde; 4-Isopropyl-alpha-methylhydrocinnamaldehyde; EINECS 203-161-7; Hydrocinnamaldehyde, isopropylmethyl-; 2-METHYL-3-(4-(PROPAN-2-YL)PHENYL)PROPANAL; BRN 2047689; UNII-4U37UX0E1E; alpha-Methyl-p-isopropylhydrocinnamaldehyde; p-Isopropyl-alpha-methylhydrocinnamaldehyde; AI3-03941; alpha-Methyl-p-isopropylphenylpropylaldehyde; p-Isopropyl-alpha-methyl-hydrocinnamaldehyde; 4-Isopropyl-alpha-methylhydrocinnamic aldehyde; alpha-Methyl-4-(1-methylethyl)benzenepropanal; p-Isopropyl-alpha-methylhydrocinnamic aldehyde; Benzenepropanal, alpha-methyl-4-(1-methylethyl)-; HYDROCINNAMALDEHYDE, p-ISOPROPYL-alpha-METHYL-; CYCLAMENALDEHYDE; EC 203-161-7; SCHEMBL1148; DSSTox_CID_24769; DSSTox_RID_80460; DSSTox_GSID_44769; 4-07-00-00788 (Beilstein Handbook Reference); CYCLAMEN ALDEHYDE [FCC]; CHEMBL3183483; CYCLAMEN ALDEHYDE [INCI]; DTXSID2044769; CHEBI:188336; AMY11259; Tox21_301084; MFCD00024160; 3-(p-Isopropylphenyl)isobutyraldehyde; AKOS009157690; NCGC00248282-01; NCGC00254985-01; 2-methyl-3-(4-isopropylphenyl)-propanal; CAS-103-95-7; 3-(4-Isopropylphenyl)-2-methylpropanal #; DB-021664; .alpha.-Methyl-p-isopropylhydrocinnamaldehyde; CS-0119970; FT-0613680; I0377; p-Isopropyl-.alpha.-methylhydrocinnamaldehyde; 2-methyl-3-(4-isopropylphenyl)propionaldehyde; 2-methyl-3-(4-isopropylphenyl)-propionaldehyde; p-Isopropyl-.alpha.-methylphenylpropyl aldehyde; 3-(4-isopropyl-phenyl)-2-methyl-propionaldehyde; Hydrocinnamaldehyde, p-isopropyl-.alpha.-methyl-; p-Isopropyl-.alpha.-methylhydrocinnamic aldehyde; .beta.-Methyl-p-iso-propyl phenyl propionaldehyde; W-109384; P-ISOPROPYL-.ALPHA.-METHYL-HYDROCINNAMALDEHYDE; Q19903910; 2-METHYL-3-(P- ISOPROPYLPHENYL)-PROPIONALDE-HYDE; 2-Methyl-3-(p-isopropylphenyl)propionaldehyde(Cyclamal); .ALPHA.-METHYL-P- ISOPROPYLHYDRO- CINNAMAL-DEHYDE; 2-METHYL-3-(P-ISOPROPYLPHENYL)PROPIONALDEHYDE [FHFI]; P-ISOPROPYL-.ALPHA.-METHYL-HYDROCINNAMALDEHYDE, (+/-)-; 2-Methyl-3-(p-isopropylphenyl)propionaldehyde, >=95%, FCC, FG; BENZENEPROPANAL, .ALPHA.-METHYL-4-(1-METHYLETHYL)-, (+/-)-
|
|
| CAS | 103-95-7 | |
| PubChem CID | 517827 | |
| ChEMBL ID | CHEMBL3183483 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 190.28 | ALogp: | 3.3 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.657 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.435 | MDCK Permeability: | 0.00001970 |
| Pgp-inhibitor: | 0.013 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.243 |
| 30% Bioavailability (F30%): | 0.5 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.94 | Plasma Protein Binding (PPB): | 63.89% |
| Volume Distribution (VD): | 3.236 | Fu: | 28.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.664 | CYP1A2-substrate: | 0.885 |
| CYP2C19-inhibitor: | 0.636 | CYP2C19-substrate: | 0.859 |
| CYP2C9-inhibitor: | 0.323 | CYP2C9-substrate: | 0.433 |
| CYP2D6-inhibitor: | 0.898 | CYP2D6-substrate: | 0.758 |
| CYP3A4-inhibitor: | 0.167 | CYP3A4-substrate: | 0.555 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.339 | Half-life (T1/2): | 0.335 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.057 | Human Hepatotoxicity (H-HT): | 0.045 |
| Drug-inuced Liver Injury (DILI): | 0.558 | AMES Toxicity: | 0.067 |
| Rat Oral Acute Toxicity: | 0.012 | Maximum Recommended Daily Dose: | 0.041 |
| Skin Sensitization: | 0.96 | Carcinogencity: | 0.356 |
| Eye Corrosion: | 0.93 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.086 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000026 | 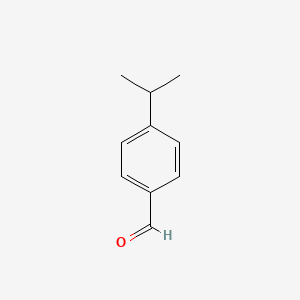 |
0.545 | D0R1QE | 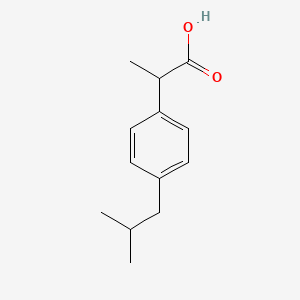 |
0.560 | ||
| ENC000199 | 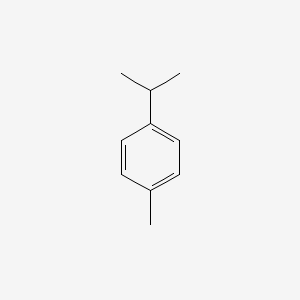 |
0.512 | D0YQ5L | 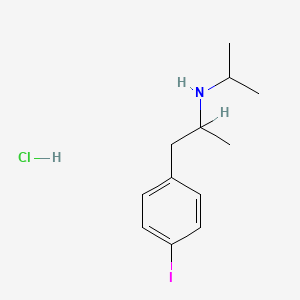 |
0.453 | ||
| ENC000368 | 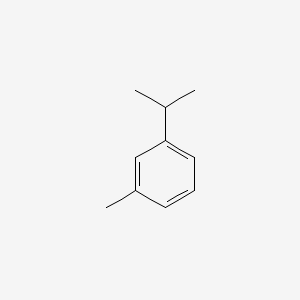 |
0.354 | D0W1RY | 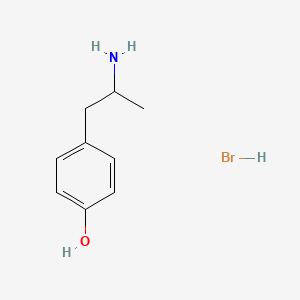 |
0.380 | ||
| ENC000619 | 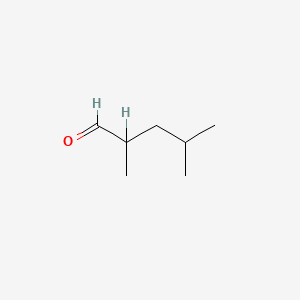 |
0.349 | D08GYO | 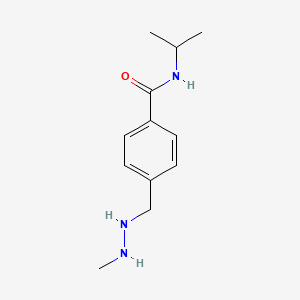 |
0.344 | ||
| ENC000191 | 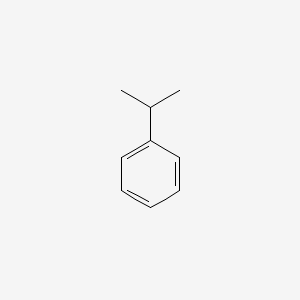 |
0.340 | D04VMT | 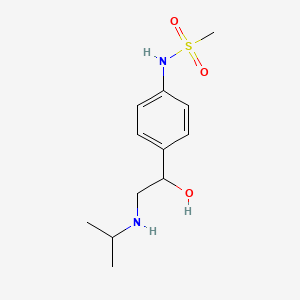 |
0.344 | ||
| ENC000347 | 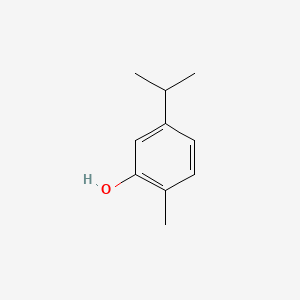 |
0.340 | D06YPU | 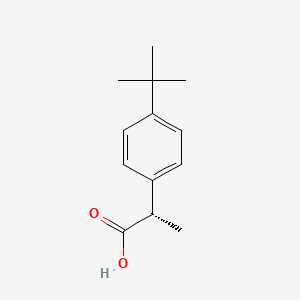 |
0.328 | ||
| ENC000796 | 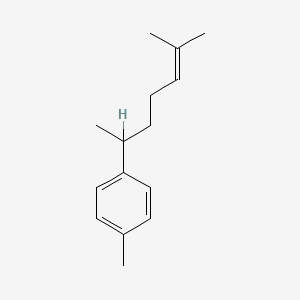 |
0.339 | D01CRB | 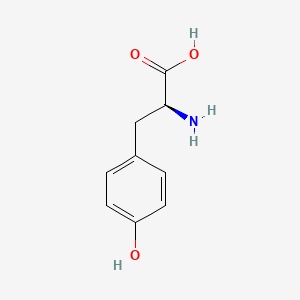 |
0.327 | ||
| ENC000098 | 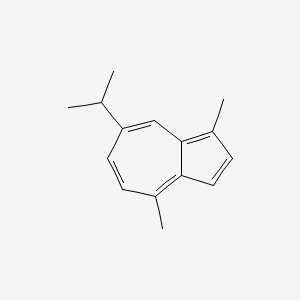 |
0.339 | D04KJO | 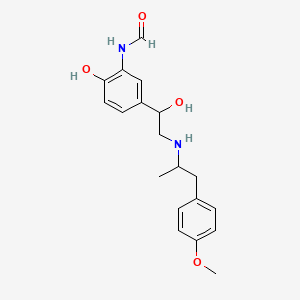 |
0.317 | ||
| ENC000638 | 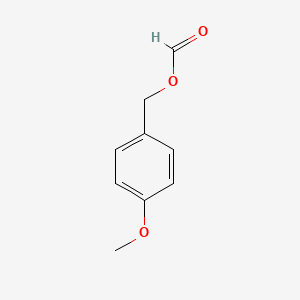 |
0.333 | D0D1DI | 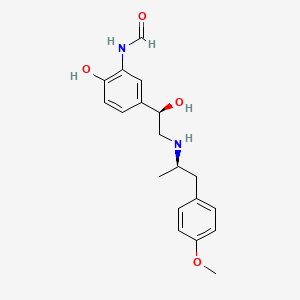 |
0.317 | ||
| ENC000129 | 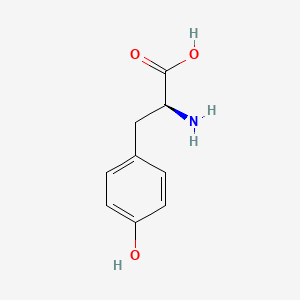 |
0.327 | D0Q1IT | 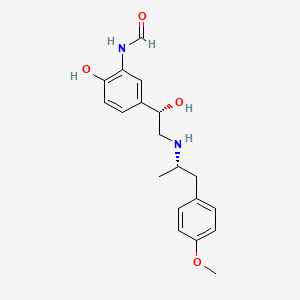 |
0.317 | ||