NPs Basic Information
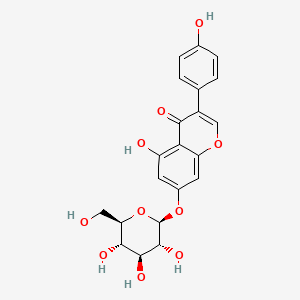
|
Name |
Genistin
|
| Molecular Formula | C21H20O10 | |
| IUPAC Name* |
5-hydroxy-3-(4-hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one
|
|
| SMILES |
C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)O
|
|
| InChI |
InChI=1S/C21H20O10/c22-7-15-18(26)19(27)20(28)21(31-15)30-11-5-13(24)16-14(6-11)29-8-12(17(16)25)9-1-3-10(23)4-2-9/h1-6,8,15,18-24,26-28H,7H2/t15-,18-,19+,20-,21-/m1/s1
|
|
| InChIKey |
ZCOLJUOHXJRHDI-CMWLGVBASA-N
|
|
| Synonyms |
Genistin; 529-59-9; Genistoside; Genistine; Genistein 7-glucoside; Genistein glucoside; Genisteol 7-monoglucoside; Genistein 7-O-beta-D-glucoside; NSC 5112; Glucosyl-7-genistein; CHEBI:27514; genistein 7-O-glucoside; 1POG3SCN5T; 5-hydroxy-3-(4-hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one; Genistein, 7-O-beta-D-glucoside; 4',5,7-trihydroxyisoflavone 7-D-glucoside; Genistein, 7-beta-D-glucopyranoside; Isoflavone, 4',5,7-trihydroxy-, 7-D-glucoside; 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl beta-D-glucopyranoside; 5-hydroxy-3-(4-hydroxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one; 4',5,7-Trihydroxyisoflavone 7-.beta.-D-glucopyranoside; 4',5,7-Trihydroxyisoflavone 7-glucoside; UNII-1POG3SCN5T; Glucopyranoside, genistein-7, beta-D-; BRN 0064479; NSC-5112; 7-O-.beta.-D-glucopyranosyl genistein; Genistein glycoside; Genistin,(S); 5-hydroxy-3-(4-hydroxyphenyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one; MFCD00016883; GENISTIN [USP-RS]; SCHEMBL62148; Genistin, analytical standard; 4-18-00-02732 (Beilstein Handbook Reference); MLS006010735; BIDD:ER0520; CHEMBL486625; MEGxp0_000436; GENISTEIN GENISTIN [MI]; DTXSID3022324; ACon1_001495; Genistin - CAS 529-59-9; Genistin, >=97.5% (TLC); Genistein 7-O-b-D-glucopyranoside; HY-N0595; ZINC4097913; BDBM50025609; AKOS025146960; CCG-208377; CS-4240; 4H-1-Benzopyran-4-one, 7-(beta-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-; NCGC00163559-01; NCGC00163559-02; NCGC00180447-01; AS-11684; SMR003929926; G0358; C09126; GENISTEIN 7-O-.BETA.-D-GLUCOPYRANOSIDE; Genistin, primary pharmaceutical reference standard; Q-100603; Q5533221; BRD-K51391729-001-01-4; GENISTIN (CONSTITUENT OF SOY ISOFLAVONES) [DSC]; Genistin, from Glycine max (soybean), >=95% (HPLC); 5,4'-DI-HYDROXYISOFLAVONE-7-O-.BETA.-D-GLUCOPYRANOSIDE; Genistin, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 529-59-9 | |
| PubChem CID | 5281377 | |
| ChEMBL ID | CHEMBL486625 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 432.4 | ALogp: | 0.9 |
| HBD: | 6 | HBA: | 10 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 166.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 31 | QED Weighted: | 0.34 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.314 | MDCK Permeability: | 0.00000949 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.486 |
| Human Intestinal Absorption (HIA): | 0.282 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.032 | Plasma Protein Binding (PPB): | 93.41% |
| Volume Distribution (VD): | 0.751 | Fu: | 5.36% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.234 | CYP1A2-substrate: | 0.045 |
| CYP2C19-inhibitor: | 0.037 | CYP2C19-substrate: | 0.055 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.443 |
| CYP2D6-inhibitor: | 0.508 | CYP2D6-substrate: | 0.23 |
| CYP3A4-inhibitor: | 0.094 | CYP3A4-substrate: | 0.027 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.061 | Half-life (T1/2): | 0.755 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.158 | Human Hepatotoxicity (H-HT): | 0.065 |
| Drug-inuced Liver Injury (DILI): | 0.68 | AMES Toxicity: | 0.577 |
| Rat Oral Acute Toxicity: | 0.07 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.743 | Carcinogencity: | 0.414 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.221 |
| Respiratory Toxicity: | 0.028 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004476 | 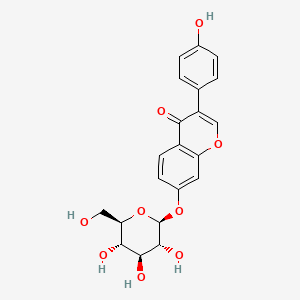 |
0.771 | D0TC7C | 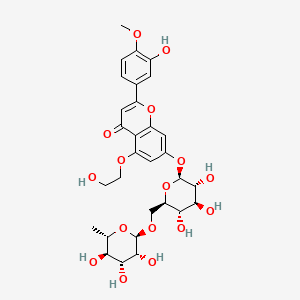 |
0.401 | ||
| ENC004734 | 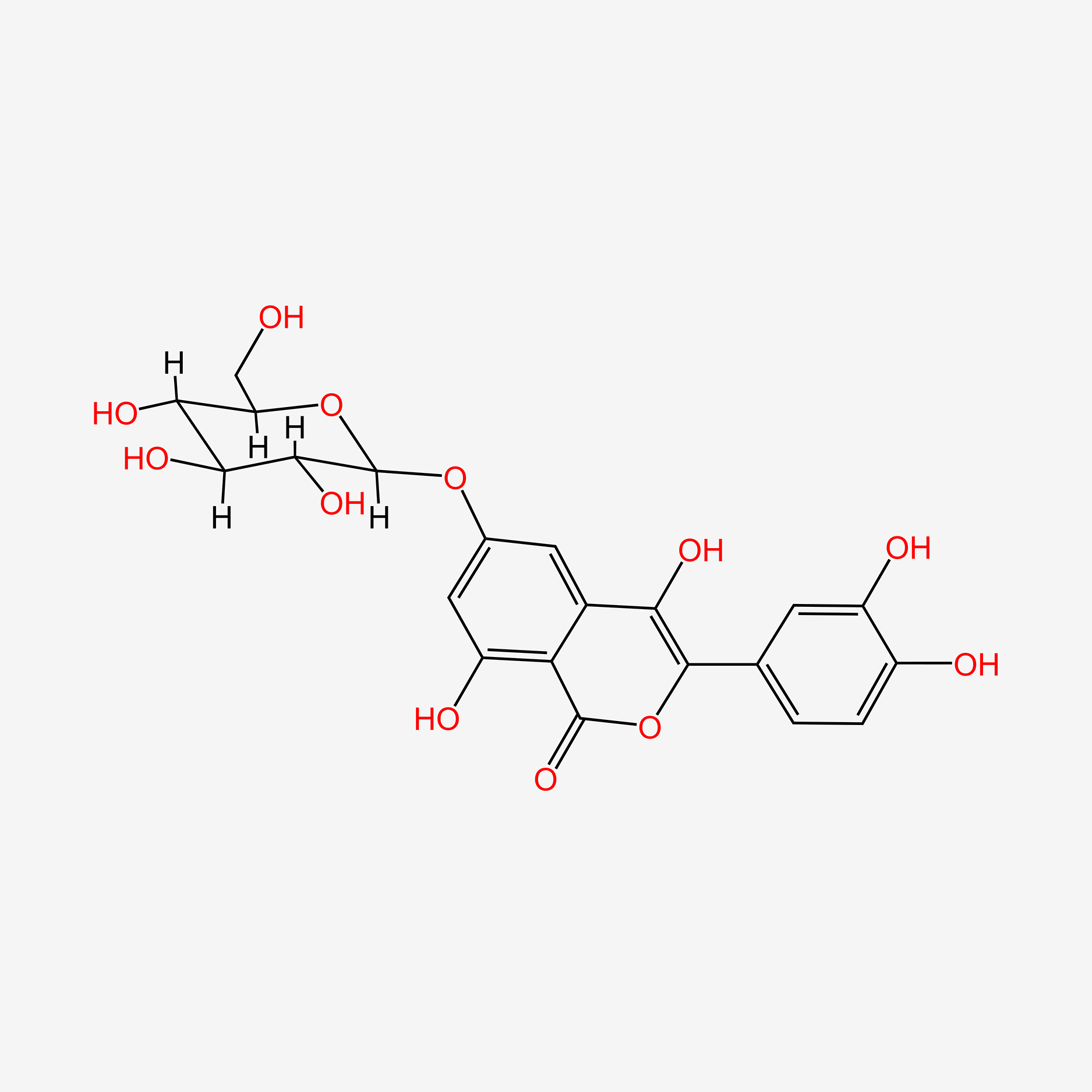 |
0.571 | D06BQU |  |
0.396 | ||
| ENC001532 |  |
0.569 | D0I9HF |  |
0.378 | ||
| ENC001550 |  |
0.560 | D08DFX |  |
0.373 | ||
| ENC004797 |  |
0.520 | D01TNW |  |
0.367 | ||
| ENC001572 |  |
0.508 | D06ALD |  |
0.359 | ||
| ENC001771 |  |
0.495 | D0K8KX |  |
0.327 | ||
| ENC002201 |  |
0.480 | D0AZ8C |  |
0.324 | ||
| ENC004073 |  |
0.454 | D04AIT |  |
0.309 | ||
| ENC001575 |  |
0.454 | D09LBS |  |
0.306 | ||