NPs Basic Information
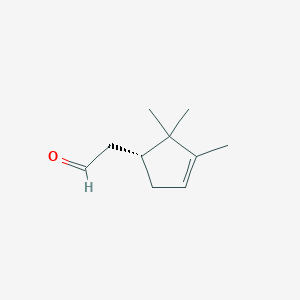
|
Name |
alpha-Campholenal
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
2-[(1R)-2,2,3-trimethylcyclopent-3-en-1-yl]acetaldehyde
|
|
| SMILES |
CC1=CC[C@@H](C1(C)C)CC=O
|
|
| InChI |
InChI=1S/C10H16O/c1-8-4-5-9(6-7-11)10(8,2)3/h4,7,9H,5-6H2,1-3H3/t9-/m1/s1
|
|
| InChIKey |
OGCGGWYLHSJRFY-SECBINFHSA-N
|
|
| Synonyms |
alpha-Campholenal; 4501-58-0; 2-[(1R)-2,2,3-trimethylcyclopent-3-en-1-yl]acetaldehyde; (R)-(+)-campholenic aldehyde; [(1R)-2,2,3-trimethylcyclopent-3-en-1-yl]acetaldehyde; (+)-campholenic aldehyde; (R)-alpha-campholenaldehyde; FEMA No. 3592; 3-Cyclopentene-1-acetaldehyde, 2,2,3-trimethyl-, (1R)-; CHEBI:49150; 75LU5216DI; (R)-2,2,3-Trimethyl-3-cyclopentene-1-acetaldehyde; 3-Cyclopentene-1-acetaldehyde, 2,2,3-trimethyl-, (R)-; alpha-Campholene aldehyde; (R)-2,2,3-Trimethylcyclopent-3-ene-1-acetaldehyde; (R)-.alpha.-Campholenic aldehyde; ((1R)-2,2,3-TRIMETHYLCYCLOPENT-3-EN-1-YL)ACETALDEHYDE; Campholenal, alpha-; (R)--campholenaldehyde; (+)-CAMPHOLENAL; Campholenaldehyde, alpha-; (R)-2-(2,2,3-Trimethylcyclopent-3-en-1-yl)acetaldehyde; DSSTox_CID_24756; DSSTox_RID_80449; DSSTox_GSID_44756; (+)-CAMPHOLENALDEHYDE; UNII-75LU5216DI; CAMPHOLENAL, .ALPHA.-; CHEMBL3184714; DTXSID3044756; SCHEMBL15316770; (+)-(R)-CAMPHOLENALDEHYDE; ZINC1063075; EINECS 224-815-8; Tox21_301648; AKOS006239754; (+)-.ALPHA.-CAMPHOLENALDEHYDE; 2,2,3-Trimethyl-3-cyclopentacetaldehyde; NCGC00256080-01; (+)-.ALPHA.-CAMPHOLENIC ALDEHYDE; CAS-4501-58-0; AI3-23129; (+)-(R)-.ALPHA.-CAMPHOLENIC ALDEHYDE; EC 224-815-8; EN300-6770905; Q27121498; 2,2,3-Trimethyl-3-cyclopentene-1-acetaldehyde, (R)-; Z1198153130; 2,2,3-TRIMETHYLCYCLOPENT-3-EN-1-YL ACETALDEHYDE [FHFI]
|
|
| CAS | 4501-58-0 | |
| PubChem CID | 1252759 | |
| ChEMBL ID | CHEMBL3184714 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 1.9 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.438 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.5 | MDCK Permeability: | 0.00001880 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.242 |
| 30% Bioavailability (F30%): | 0.009 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.97 | Plasma Protein Binding (PPB): | 54.36% |
| Volume Distribution (VD): | 2.69 | Fu: | 46.19% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.137 | CYP1A2-substrate: | 0.377 |
| CYP2C19-inhibitor: | 0.084 | CYP2C19-substrate: | 0.805 |
| CYP2C9-inhibitor: | 0.032 | CYP2C9-substrate: | 0.702 |
| CYP2D6-inhibitor: | 0.022 | CYP2D6-substrate: | 0.463 |
| CYP3A4-inhibitor: | 0.037 | CYP3A4-substrate: | 0.273 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.743 | Half-life (T1/2): | 0.364 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.004 | Human Hepatotoxicity (H-HT): | 0.065 |
| Drug-inuced Liver Injury (DILI): | 0.033 | AMES Toxicity: | 0.064 |
| Rat Oral Acute Toxicity: | 0.035 | Maximum Recommended Daily Dose: | 0.457 |
| Skin Sensitization: | 0.955 | Carcinogencity: | 0.569 |
| Eye Corrosion: | 0.993 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.94 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000847 | 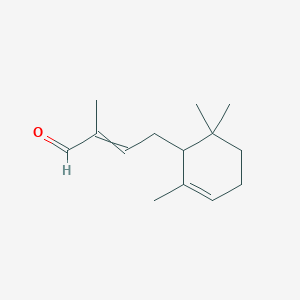 |
0.327 | D0H1QY |  |
0.208 | ||
| ENC000153 | 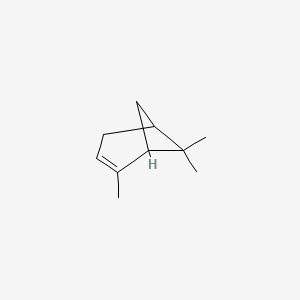 |
0.302 | D0U4VT | 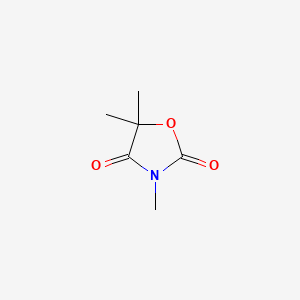 |
0.174 | ||
| ENC000574 | 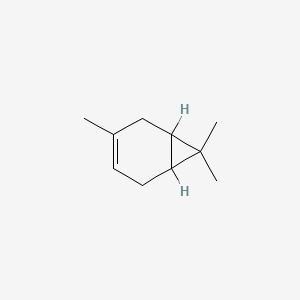 |
0.302 | D0Q4XQ | 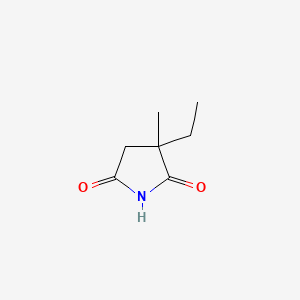 |
0.170 | ||
| ENC000636 | 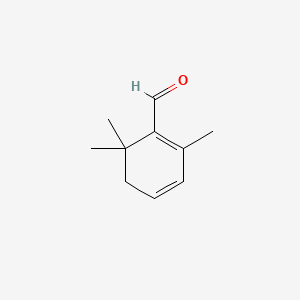 |
0.289 | D05OQJ | 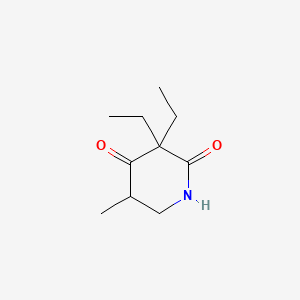 |
0.167 | ||
| ENC001898 |  |
0.261 | D0K7LU |  |
0.164 | ||
| ENC000328 |  |
0.261 | D08BYK |  |
0.164 | ||
| ENC000704 |  |
0.250 | D09JBP |  |
0.163 | ||
| ENC000146 |  |
0.250 | D0B4RU |  |
0.163 | ||
| ENC000830 |  |
0.250 | D0A2AJ |  |
0.162 | ||
| ENC001827 |  |
0.250 | D0H6VY |  |
0.161 | ||