NPs Basic Information
|
Name |
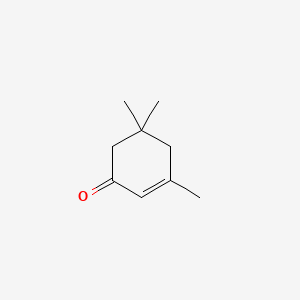
Isophorone
|
| Molecular Formula | C9H14O | |
| IUPAC Name* |
3,5,5-trimethylcyclohex-2-en-1-one
|
|
| SMILES |
CC1=CC(=O)CC(C1)(C)C
|
|
| InChI |
InChI=1S/C9H14O/c1-7-4-8(10)6-9(2,3)5-7/h4H,5-6H2,1-3H3
|
|
| InChIKey |
HJOVHMDZYOCNQW-UHFFFAOYSA-N
|
|
| Synonyms |
ISOPHORONE; 78-59-1; Isoacetophorone; 3,5,5-Trimethylcyclohex-2-en-1-one; Isoforone; 3,5,5-Trimethyl-2-cyclohexen-1-one; 3,5,5-Trimethylcyclohex-2-enone; Isooctopherone; Isoforon; Izoforon; 2-Cyclohexen-1-one, 3,5,5-trimethyl-; Isophoron; alpha-Isophorone; .alpha.-Isophoron; 1,1,3-Trimethyl-3-cyclohexene-5-one; 3,5,5-Trimethyl-2-cyclohexenone; .alpha.-Isophorone; NCI-C55618; 3,5,5-Trimethyl-2-cyclohexen-1-on; FEMA No. 3553; 3,5,5-Trimetil-2-cicloesen-1-one; Isophorone, 97%; NSC 403657; 3,5,5-Trimethyl-2-cyclohexene-1-one; 2BR99VR6WA; CHEBI:34800; NSC4881; 3,5,5-Trimethylcyclohexen-2-one-1; 3,3,5-Trimethyl-2-cyclohexen-1-one; NSC-403657; DSSTox_CID_759; DSSTox_RID_75774; DSSTox_GSID_20759; Izoforon [Polish]; 3,5-Trimethyl-2-cyclohexenone; Isoforone [Italian]; Caswell No. 506; 3,5-Trimetil-2-cicloesen-1-one; 3,5-Trimethyl-2-cyclohexen-1-one; 1,3-Trimethyl-3-cyclohexene-5-one; 3,5-Trimethyl-2-cyclohexene-1-one; WLN: L6V BUTJ C1 D1 D1; 2-Cyclohexen-1-one,5,5-trimethyl-; CAS-78-59-1; CCRIS 1353; HSDB 619; ISOPHORONE, REAG; EINECS 201-126-0; 3,5-Trimethyl-2-cyclohexen-1-on (GERMAN, DUTCH); UNII-2BR99VR6WA; EPA Pesticide Chemical Code 047401; BRN 1280721; 3,5,5-Trimethylcyclohexenone; a-Isophorone; AI3-00046; 3,5,5-Trimethylcyclohexen one; alpha -isophoron; alpha -isophorone; 3,5,5-Trimetil-2-cicloesen-1-one [Italian]; ISOACETOPHORON; nchem.180-comp3; 3,5,5-Trimethyl-2-cyclohexen-1-on [German, Dutch]; 1,5,5-Trimethyl-1-cyclohexen-3-one; ISOPHORONE [MI]; Isophorone Reagent Grade; ISOPHORONE [FHFI]; ISOPHORONE [HSDB]; EC 201-126-0; SCHEMBL22522; Isophorone, >=97%, FG; 4-07-00-00165 (Beilstein Handbook Reference); BIDD:ER0627; Isophorone, analytical standard; CHEMBL1882894; DTXSID8020759; FEMA 3553; 3,5,5-trimethyl-cyclohex-2-enone; HY-Y0932; Isophorone-2,4,4,6,6-[d5]; NSC-4881; Tox21_202312; Tox21_300050; BBL027346; MFCD00001584; NSC403657; s2998; STK801792; ZINC14822379; AKOS000120392; 3,5,5-trimethylcyclohex-2-ene-1-one; 3,5,5-trimethylcyclohexa-2-en-1-one; 3,3,5-trimethyl-cyclohex-5-en-1-one; 3,5,5-trimethyl-cyclohex-2-en-1-one; 1,1, 3-Trimethyl-3-cyclohexene-5-one; 1,3,3-TRIMETHYLCYLOHEXEN-5-ONE; 3,5, 5-Trimethyl-2-cyclohexene-1-one; NCGC00164006-01; NCGC00164006-02; NCGC00164006-03; NCGC00254115-01; NCGC00259861-01; 3,3,5-trimethyl-cyclohex-5 -en-1-one; AC-10580; VS-08530; 1,5,5-TRIMETHYL-3-OXOCYCLOHEXENE; Isophorone, Vetec(TM) reagent grade, 97%; CS-0015924; FT-0627443; I0151; EN300-20384; D72515; A839454; Q415519; W-104274; F0001-2053
|
|
| CAS | 78-59-1 | |
| PubChem CID | 6544 | |
| ChEMBL ID | CHEMBL1882894 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.21 | ALogp: | 1.6 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.502 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.563 | MDCK Permeability: | 0.00003010 |
| Pgp-inhibitor: | 0.047 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.959 | Plasma Protein Binding (PPB): | 69.41% |
| Volume Distribution (VD): | 0.73 | Fu: | 47.94% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.145 | CYP1A2-substrate: | 0.751 |
| CYP2C19-inhibitor: | 0.801 | CYP2C19-substrate: | 0.895 |
| CYP2C9-inhibitor: | 0.276 | CYP2C9-substrate: | 0.902 |
| CYP2D6-inhibitor: | 0.017 | CYP2D6-substrate: | 0.866 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.292 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.648 | Half-life (T1/2): | 0.795 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.309 |
| Drug-inuced Liver Injury (DILI): | 0.173 | AMES Toxicity: | 0.013 |
| Rat Oral Acute Toxicity: | 0.06 | Maximum Recommended Daily Dose: | 0.075 |
| Skin Sensitization: | 0.908 | Carcinogencity: | 0.861 |
| Eye Corrosion: | 0.903 | Eye Irritation: | 0.947 |
| Respiratory Toxicity: | 0.544 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001898 | 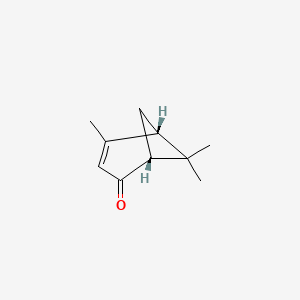 |
0.341 | D0H1QY |  |
0.250 | ||
| ENC001538 | 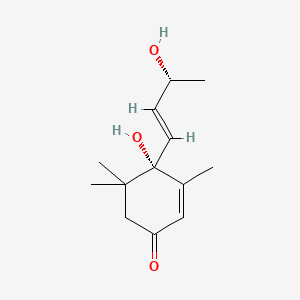 |
0.340 | D0U4VT | 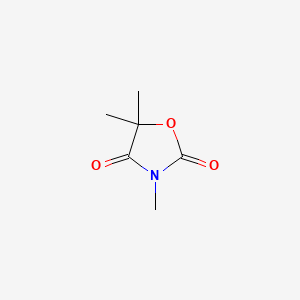 |
0.244 | ||
| ENC005579 | 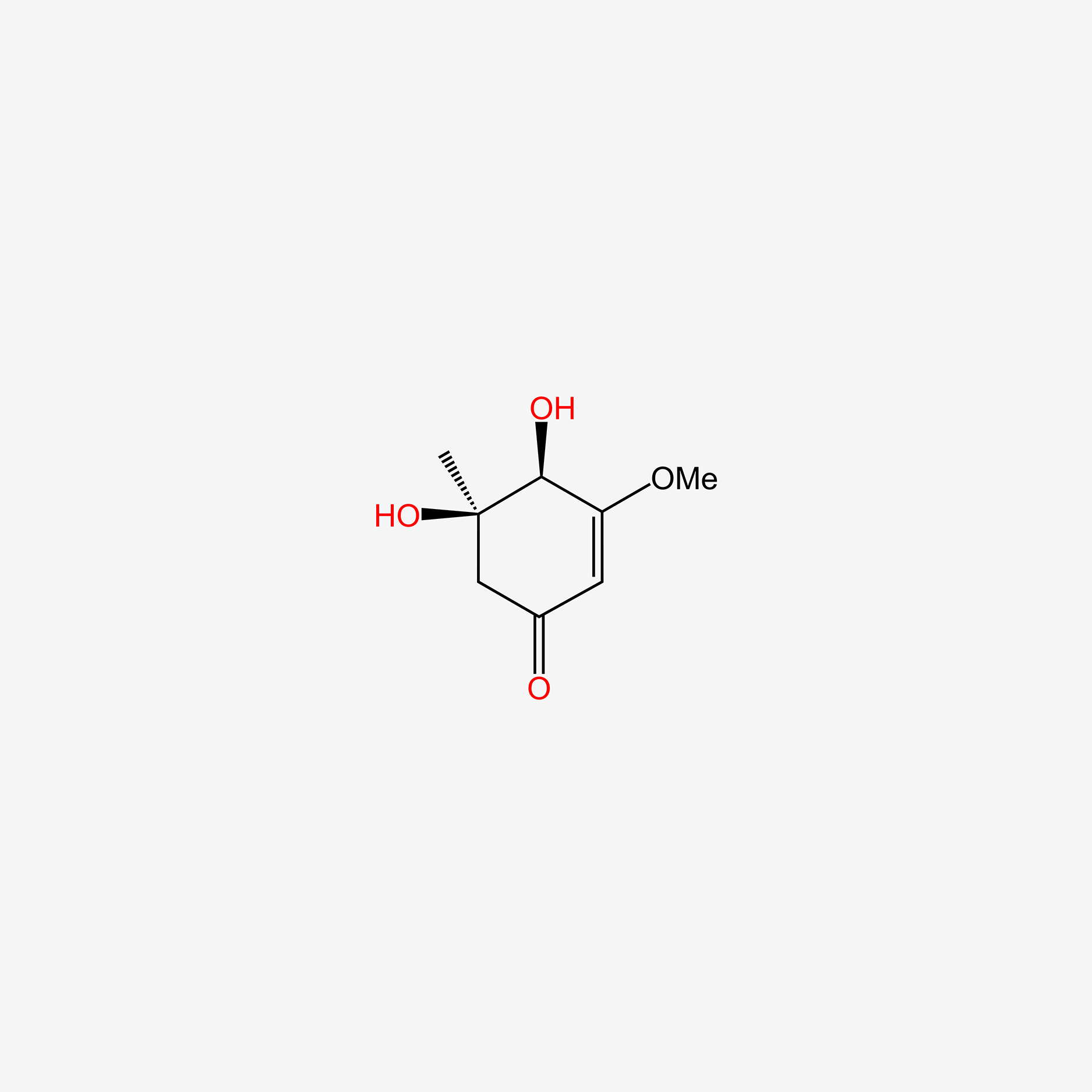 |
0.326 | D0Q4XQ | 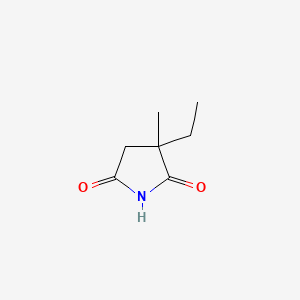 |
0.238 | ||
| ENC000457 | 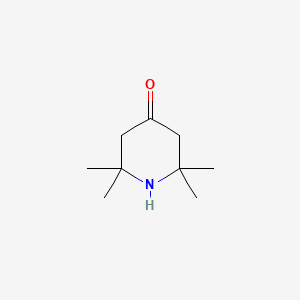 |
0.317 | D0K7LU | 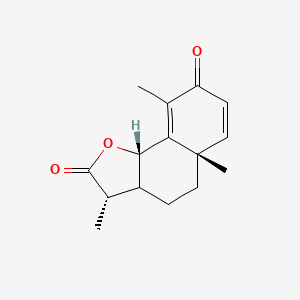 |
0.210 | ||
| ENC005034 |  |
0.310 | D0Z1XD |  |
0.208 | ||
| ENC000165 |  |
0.310 | D06XWB |  |
0.206 | ||
| ENC001332 | 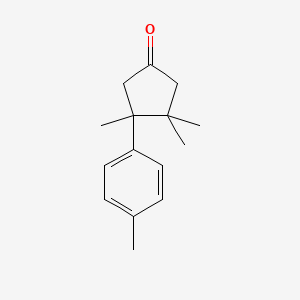 |
0.302 | D04GJN | 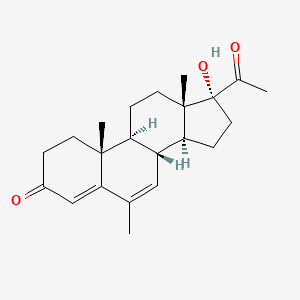 |
0.205 | ||
| ENC005108 | 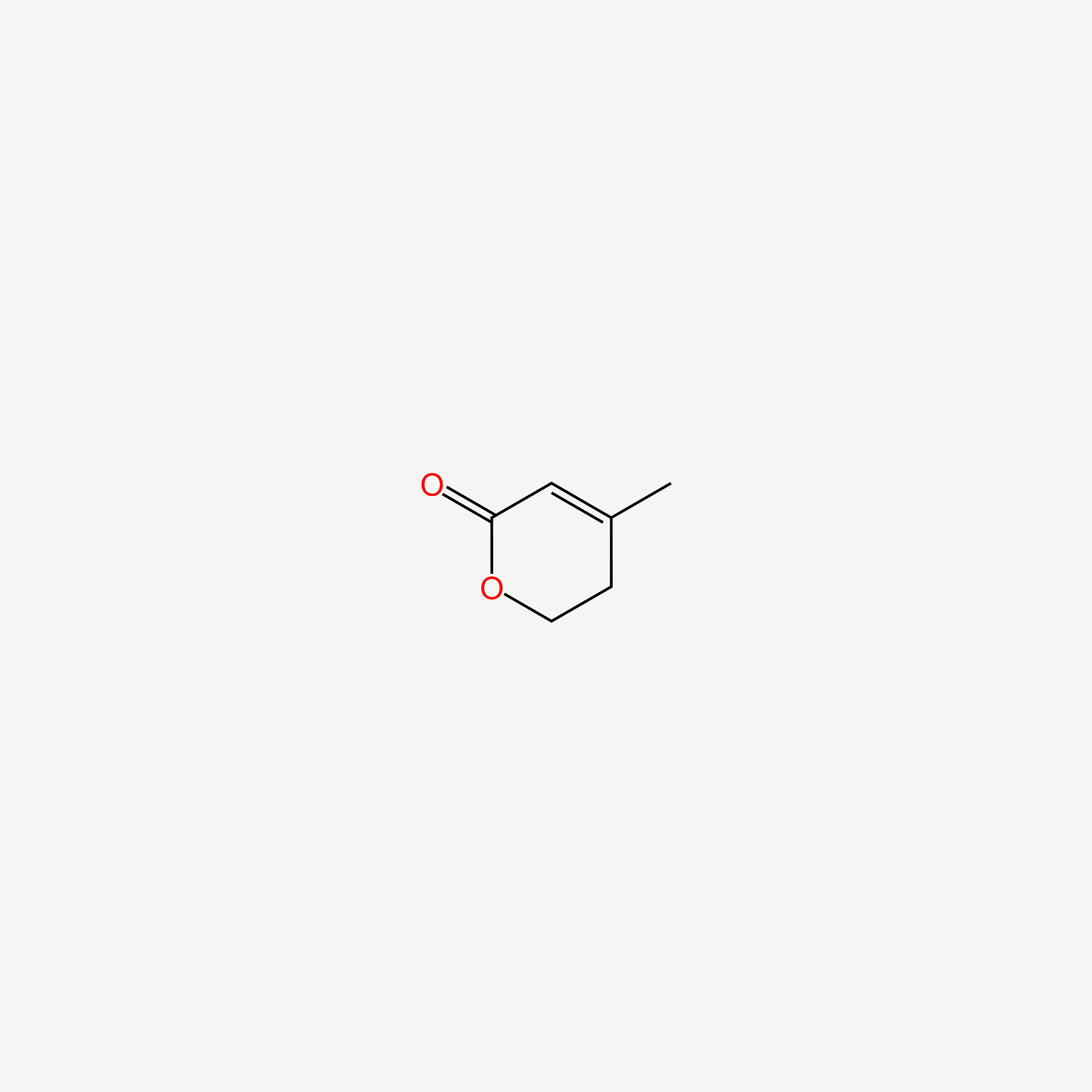 |
0.297 | D0F1UL | 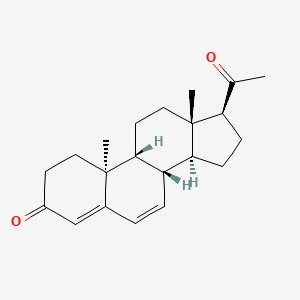 |
0.200 | ||
| ENC001280 | 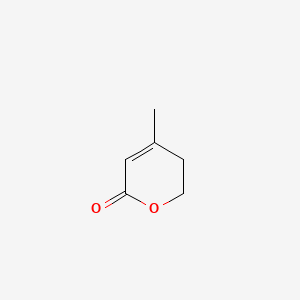 |
0.297 | D07BSQ | 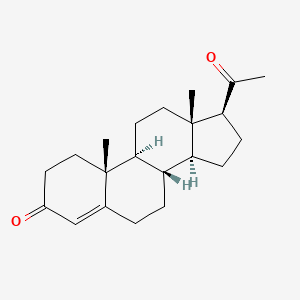 |
0.200 | ||
| ENC000574 | 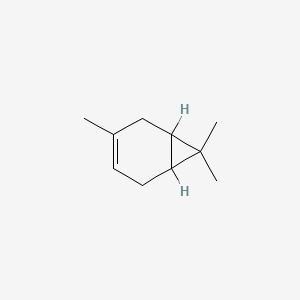 |
0.293 | D09JBP | 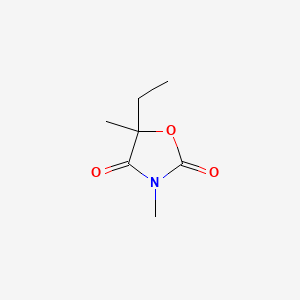 |
0.200 | ||