NPs Basic Information
|
Name |
alpha-PINENE
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
|
|
| SMILES |
CC1=CCC2CC1C2(C)C
|
|
| InChI |
InChI=1S/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h4,8-9H,5-6H2,1-3H3
|
|
| InChIKey |
GRWFGVWFFZKLTI-UHFFFAOYSA-N
|
|
| Synonyms |
ALPHA-PINENE; 80-56-8; 2-Pinene; Acintene A; .alpha.-Pinene; 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene; pin-2(3)-ene; Sylvapine A; (+/-)-2-Pinene; Pinene isomer; (+/-)-alpha-Pinene; 4,6,6-trimethylbicyclo[3.1.1]hept-3-ene; Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl-; PINENE, ALPHA; alfa-Pinene; CHEBI:36740; NSC-7727; NSC94522; NSC94523; NCGC00090682-01; NSC 7727; DSSTox_CID_6501; DSSTox_RID_78126; DSSTox_GSID_26501; 2,6,6-Trimethylbicyclo(3.1.1)-2-hept-2-ene; 1S-.alpha.-Pinene; Leavo-95; CAS-80-56-8; DL-ALPHA-PINENE; 4,6,6-Trimethylbicyklo(3,1,1)hept-3-en; (1S)-(-)-alpha-Pinene; PC-500(TERPENE); alphapinene; alpha pinene; an alpha-pinene; Cyclic dexadiene; alpha -pinene; alpha.-pinene; PC-500; Acitene A; Alpha Pinene PF; (-)alpha-pinene; Pinene, .alpha.; pin-2-ene; alpha [D] Pinene; alpha [L] Pinene; (+-)-alpha-pinene; 1R-.alpha.-Pinene; 2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene; (R)-.alpha.-Pinene; (+-)-2-pinene; (-)-?-Pinene; (1R)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene; (1S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene; PINENE, ALPHA (D); PINENE, ALPHA (L); (.+/-.)-.alpha.-Pinene; CHEMBL442565; DTXSID4026501; NSC7727; alpha-Pinene (+/-)-alpha-Pinene; AMY22338; Tox21_110996; Tox21_200108; Tox21_303385; MFCD00001339; NSC-94522; NSC-94523; PC 500; AKOS000121239; AB86235; AB86464; AB93066; DB15573; UN 2368; NCGC00090682-02; NCGC00257379-01; NCGC00257662-01; 25766-18-1; LS-13835; 2,6-Trimethylbicyclo[3.1.1]-2-heptene; DB-017892; 2,6,6-Trimethylbicyclo[3.1.1]-2-heptene; alpha-Pinene 1000 microg/mL in Isopropanol; Bicyclo[3.1.1]hept-2-ene,6,6-trimethyl-; FT-0604379; FT-0604414; FT-0622197; FT-0698080; 2,6-Trimethylbicyclo[3.1.1]-2-hept-2-ene; EN300-21685; 2,6,6-Trimethyl bicyclo-(3,1,1)-2 heptene; C09880; A839247; Q-201582; (3Z)-5-METHYL-1H-INDOLE-2,3-DIONE3-OXIME; Q27104380; Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl-, (.+/-.)-; (+/-)-2-Pinene, 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene, alpha-Pinene
|
|
| CAS | 80-56-8 | |
| PubChem CID | 6654 | |
| ChEMBL ID | CHEMBL442565 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 2.8 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 10 | QED Weighted: | 0.444 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.303 | MDCK Permeability: | 0.00001830 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.102 |
| 30% Bioavailability (F30%): | 0.102 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.896 | Plasma Protein Binding (PPB): | 86.34% |
| Volume Distribution (VD): | 1.73 | Fu: | 12.59% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.469 | CYP1A2-substrate: | 0.368 |
| CYP2C19-inhibitor: | 0.267 | CYP2C19-substrate: | 0.867 |
| CYP2C9-inhibitor: | 0.312 | CYP2C9-substrate: | 0.846 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.786 |
| CYP3A4-inhibitor: | 0.045 | CYP3A4-substrate: | 0.263 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 15.022 | Half-life (T1/2): | 0.114 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.006 | Human Hepatotoxicity (H-HT): | 0.196 |
| Drug-inuced Liver Injury (DILI): | 0.023 | AMES Toxicity: | 0.002 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.42 |
| Skin Sensitization: | 0.158 | Carcinogencity: | 0.056 |
| Eye Corrosion: | 0.955 | Eye Irritation: | 0.985 |
| Respiratory Toxicity: | 0.825 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
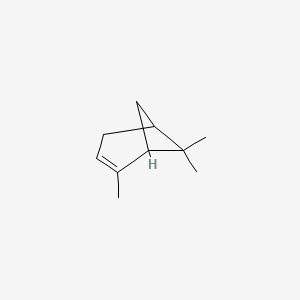
| ENC001827 | 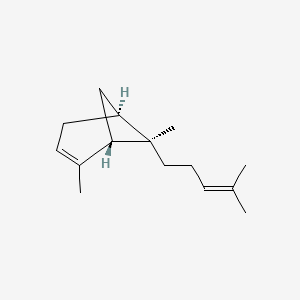 |
0.478 | D0V8HA | 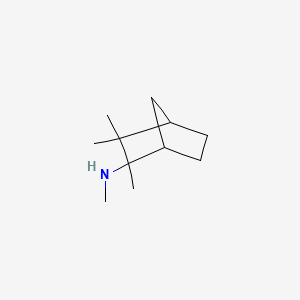 |
0.255 | ||
| ENC000830 | 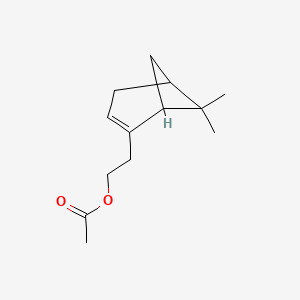 |
0.478 | D0H1QY |  |
0.244 | ||
| ENC000770 | 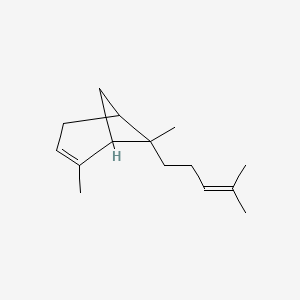 |
0.478 | D0A2AJ | 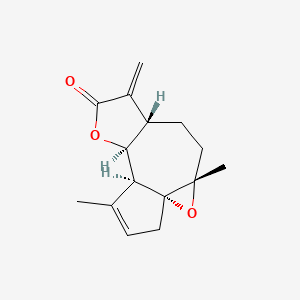 |
0.203 | ||
| ENC001898 | 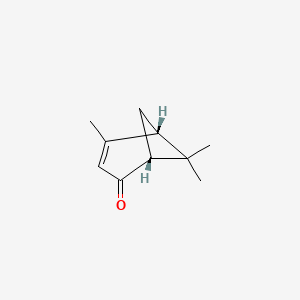 |
0.436 | D05VQI | 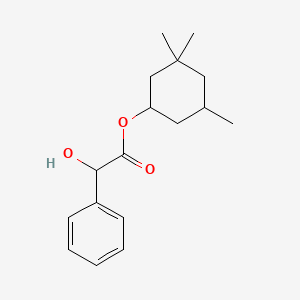 |
0.188 | ||
| ENC000613 |  |
0.421 | D0K7LU |  |
0.188 | ||
| ENC000574 |  |
0.421 | D0B4RU |  |
0.182 | ||
| ENC000482 |  |
0.421 | D0K0EK |  |
0.178 | ||
| ENC002084 |  |
0.400 | D0G6AB |  |
0.171 | ||
| ENC001831 |  |
0.360 | D0P1FO | 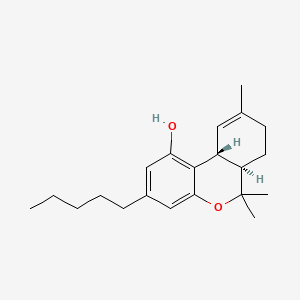 |
0.167 | ||
| ENC000520 | 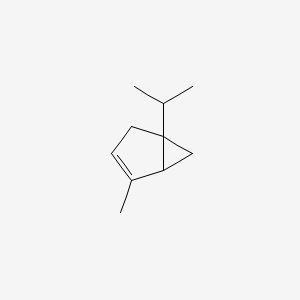 |
0.350 | D04CSZ | 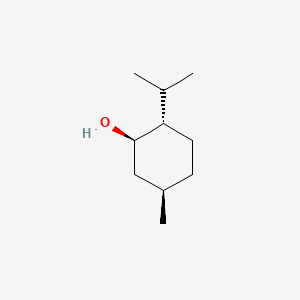 |
0.167 | ||