NPs Basic Information
|
Name |
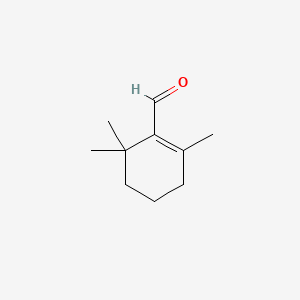
beta-Cyclocitral
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
2,6,6-trimethylcyclohexene-1-carbaldehyde
|
|
| SMILES |
CC1=C(C(CCC1)(C)C)C=O
|
|
| InChI |
InChI=1S/C10H16O/c1-8-5-4-6-10(2,3)9(8)7-11/h7H,4-6H2,1-3H3
|
|
| InChIKey |
MOQGCGNUWBPGTQ-UHFFFAOYSA-N
|
|
| Synonyms |
beta-Cyclocitral; 432-25-7; b-cyclocitral; 2,6,6-Trimethylcyclohexene-1-carbaldehyde; 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl-; 2,6,6-trimethylcyclohex-1-ene-1-carbaldehyde; .beta.-Cyclocitral; 2,6,6-TRIMETHYL-1-CYCLOHEXENE-1-CARBOXALDEHYDE; CYCLOCITRAL; alpha(beta)-Cyclocitral; 1-Formyl-2,6,6-trimethyl-1-cyclohexene; 2,6,6-trimethylcyclohex-1-enecarbaldehyde; beta -cyclocitral; 2,6,6-Trimethylcyclohexenecarbaldehyde; Cyclohexenecarboxaldehyde, 2,6,6-trimethyl-; MFCD00079078; CHEBI:53177; 2,6,6-Trimethyl-1-cyclohexen-1-carboxaldehyde; 77Y0U2X29G; beta-Cyclocitral, Technical Grade; 2,6,6-trimethyl-cyclohexene-1-carboxaldehyde; 2,6,6-Trimethyl-1-cyclohexene-1-carbaldehyde; UNII-77Y0U2X29G; beta cyclocitral; beta-cyclocitrol; EINECS 207-081-3; 2,6,6-Trimethyl-1-cyclohexenecarbaldehyde; AI3-37227; 2,6,6-Trimethyl-1-cyclohexenecarboxaldehyde; UNII-GLL5338RMI; beta-Cyclocitral, >=95%; DSSTox_CID_27142; DSSTox_RID_82149; GLL5338RMI; DSSTox_GSID_47142; CYCLOCITRAL, .BETA.-; SCHEMBL309759; CHEMBL1952257; DTXSID7047142; ZINC5766948; EINECS 258-219-4; Tox21_302524; beta-Cyclocitral, analytical standard; AKOS022504751; CS-W010947; HY-W010231; FEMA NO. 3639, .BETA.-; NCGC00256741-01; AS-56746; CAS-432-25-7; SY029933; 2,6,6-Trimethyl-Cyclohexenecarboxaldehyde; 2,6,6-trimethyl-cyclohexene-1-carbaldehyde; FT-0665336; FT-0665337; C20425; EN300-180215; F14917; 2,6,6-Trimethyl-1-cyclohexene-1-carbaldehyde #; W-202757; Q27124011; Z1255386953; Pentadeuterio-beta-cyclocitral;1-Formyl-2,6,6-trimethyl-1-cyclohexene
|
|
| CAS | 432-25-7 | |
| PubChem CID | 9895 | |
| ChEMBL ID | CHEMBL1952257 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 2.4 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.525 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.508 | MDCK Permeability: | 0.00002370 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.03 |
| 30% Bioavailability (F30%): | 0.004 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.665 | Plasma Protein Binding (PPB): | 82.47% |
| Volume Distribution (VD): | 1.63 | Fu: | 27.16% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.575 | CYP1A2-substrate: | 0.927 |
| CYP2C19-inhibitor: | 0.514 | CYP2C19-substrate: | 0.898 |
| CYP2C9-inhibitor: | 0.085 | CYP2C9-substrate: | 0.898 |
| CYP2D6-inhibitor: | 0.22 | CYP2D6-substrate: | 0.855 |
| CYP3A4-inhibitor: | 0.033 | CYP3A4-substrate: | 0.296 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.314 | Half-life (T1/2): | 0.439 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.009 | Human Hepatotoxicity (H-HT): | 0.032 |
| Drug-inuced Liver Injury (DILI): | 0.102 | AMES Toxicity: | 0.253 |
| Rat Oral Acute Toxicity: | 0.058 | Maximum Recommended Daily Dose: | 0.202 |
| Skin Sensitization: | 0.289 | Carcinogencity: | 0.486 |
| Eye Corrosion: | 0.966 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.958 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001425 | 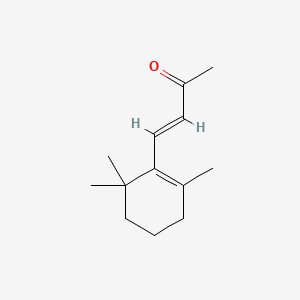 |
0.610 | D02DGU | 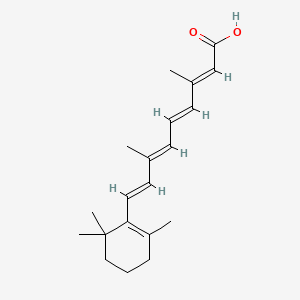 |
0.397 | ||
| ENC001738 |  |
0.438 | D00DKK | 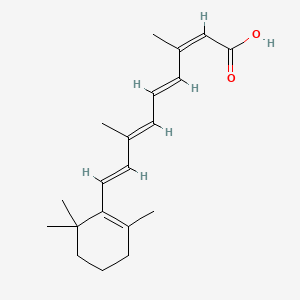 |
0.397 | ||
| ENC000636 | 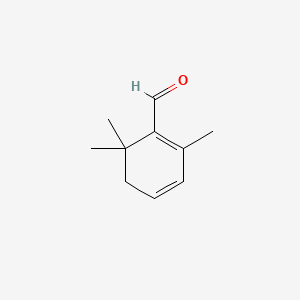 |
0.415 | D0G3PI | 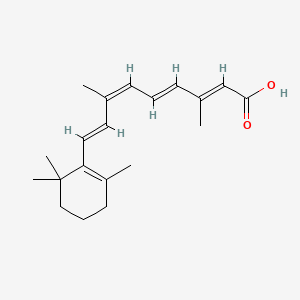 |
0.397 | ||
| ENC001830 | 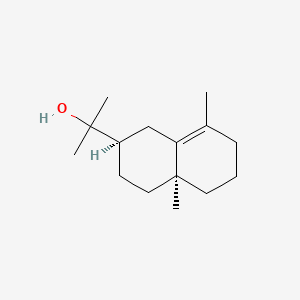 |
0.309 | D0S7WX | 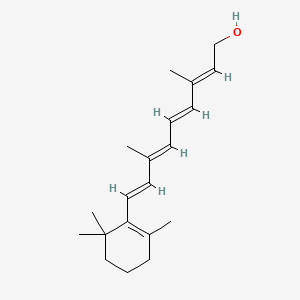 |
0.387 | ||
| ENC001609 |  |
0.298 | D0H1QY |  |
0.261 | ||
| ENC002199 |  |
0.296 | D0MY8N |  |
0.209 | ||
| ENC001739 |  |
0.296 | D0U4VT |  |
0.200 | ||
| ENC000588 | 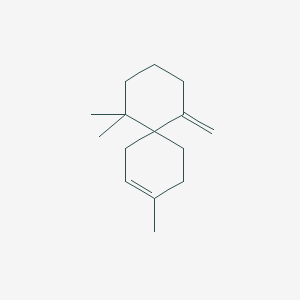 |
0.296 | D0G8BV | 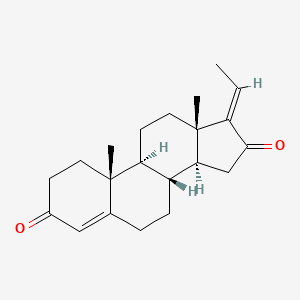 |
0.192 | ||
| ENC001341 | 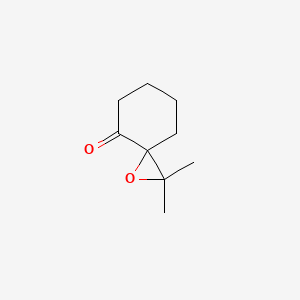 |
0.289 | D00ETS |  |
0.190 | ||
| ENC005114 | 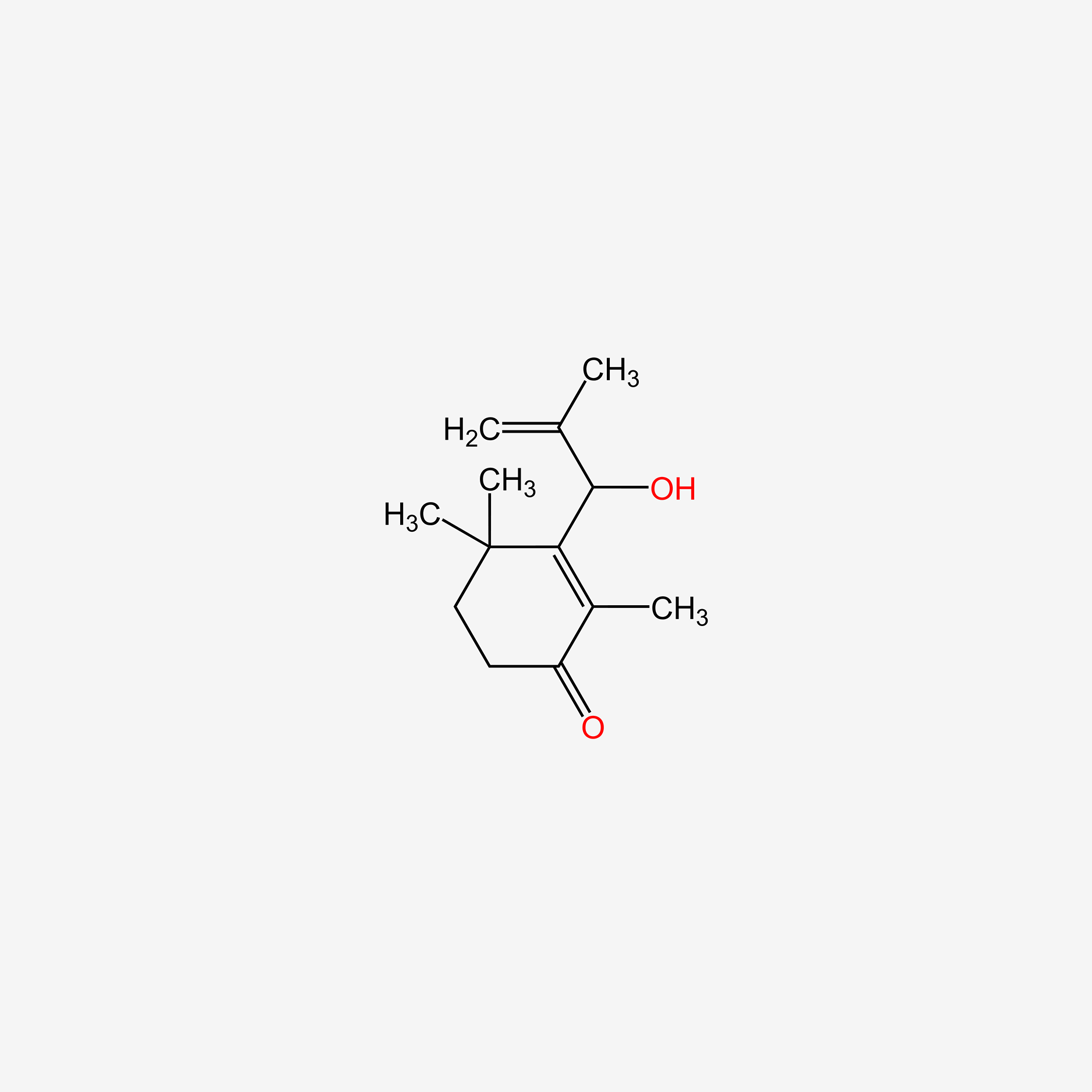 |
0.288 | D0Z1XD | 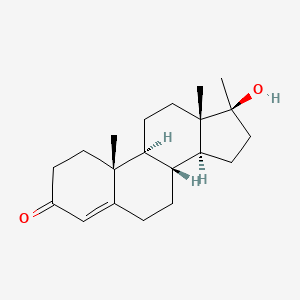 |
0.184 | ||