NPs Basic Information
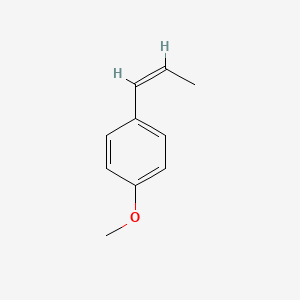
|
Name |
cis-Anethole
|
| Molecular Formula | C10H12O | |
| IUPAC Name* |
1-methoxy-4-[(Z)-prop-1-enyl]benzene
|
|
| SMILES |
C/C=C\C1=CC=C(C=C1)OC
|
|
| InChI |
InChI=1S/C10H12O/c1-3-4-9-5-7-10(11-2)8-6-9/h3-8H,1-2H3/b4-3-
|
|
| InChIKey |
RUVINXPYWBROJD-ARJAWSKDSA-N
|
|
| Synonyms |
cis-Anethole; 25679-28-1; (Z)-Anethole; (Z)-1-Methoxy-4-(prop-1-en-1-yl)benzene; cis-p-Propenylanisole; ANISOLE, p-PROPENYL-, cis-; 1-methoxy-4-[(Z)-prop-1-enyl]benzene; Benzene, 1-methoxy-4-(1-propenyl)-, (Z)-; Anethole, (Z)-; 78AWK1V4GL; CHEBI:78412; cis-p-Anethole; 1-methoxy-4-[(1Z)-prop-1-en-1-yl]benzene; (Z-)-Anethole; E-anethole; trans-p-Anethole; Anethole, cis; Methoxy-4-propenylbenzene; 4-06-00-03796 (Beilstein Handbook Reference); (E)-1-p-Methoxyphenylpropene; EINECS 247-181-4; UNII-78AWK1V4GL; BRN 1209632; trans-1-(p-Methoxyphenyl)propene; Anistearoptene; 1-(4-Methoxyphenyl)-1(3)-propene; trans-1-(p-Methoxyphenyl)-1-propene; cis-p-Methoxy-beta-methylstyrene; trans-1-(4-Methoxyphenyl)-1-propene; trans-1-Methoxy-4-(1-propenyl)benzene; Anethole, USAN; NCGC00091493-01; 1-Methoxy-4-((1E)-1-propenyl)benzene; trans-Anise camphor; 4-cis-propenyl-anisole; p-Propenylanisole, 8CI; 1-(methyloxy)-4-[(1E)-prop-1-en-1-yl]benzene; ANETHOLE, CIS-; p-Propenyl-trans-Anisole; CIS-ANETHOLE [MI]; SCHEMBL57011; ghl.PD_Mitscher_leg0.374; 1-(p-Methoxyphenyl)-Propene; CHEMBL1468832; DTXSID4058651; FEMA 2086; 1-Methoxy-4-(propenyl)-Benzene; ZINC12358735; 1-Methoxy-4-(propen-1-yl)-Benzene; AKOS015840488; Anisole, p-propenyl-, (E)- (8CI); 1-Methoxy-4-(1E)-1-propenyl-Benzene; 1-Methoxy-4-(1-propenyl)benzene, 9CI; 1-Methoxy-4-(1-propenyl)-(E)-Benzene; 1-Methoxy-4-(1E)-1-propen-1-yl-Benzene; methyl 4-[(1Z)-prop-1-en-1-yl]phenyl ether; Q27147814
|
|
| CAS | 25679-28-1 | |
| PubChem CID | 1549040 | |
| ChEMBL ID | CHEMBL1468832 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 148.2 | ALogp: | 3.3 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 9.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.623 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.231 | MDCK Permeability: | 0.00001920 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.024 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.906 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.91 | Plasma Protein Binding (PPB): | 92.97% |
| Volume Distribution (VD): | 2.455 | Fu: | 7.78% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.986 | CYP1A2-substrate: | 0.939 |
| CYP2C19-inhibitor: | 0.801 | CYP2C19-substrate: | 0.783 |
| CYP2C9-inhibitor: | 0.256 | CYP2C9-substrate: | 0.178 |
| CYP2D6-inhibitor: | 0.558 | CYP2D6-substrate: | 0.581 |
| CYP3A4-inhibitor: | 0.581 | CYP3A4-substrate: | 0.517 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.902 | Half-life (T1/2): | 0.578 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.044 | Human Hepatotoxicity (H-HT): | 0.512 |
| Drug-inuced Liver Injury (DILI): | 0.729 | AMES Toxicity: | 0.103 |
| Rat Oral Acute Toxicity: | 0.02 | Maximum Recommended Daily Dose: | 0.043 |
| Skin Sensitization: | 0.309 | Carcinogencity: | 0.608 |
| Eye Corrosion: | 0.412 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.044 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001441 | 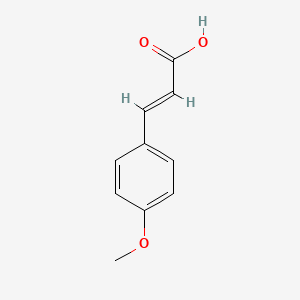 |
0.634 | D02DPU |  |
0.333 | ||
| ENC001578 | 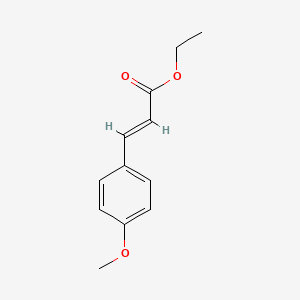 |
0.587 | D0DJ1B |  |
0.300 | ||
| ENC000221 | 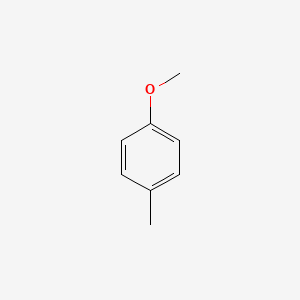 |
0.556 | D0P1UX | 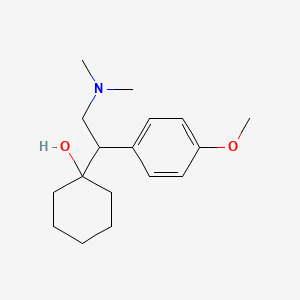 |
0.299 | ||
| ENC000318 | 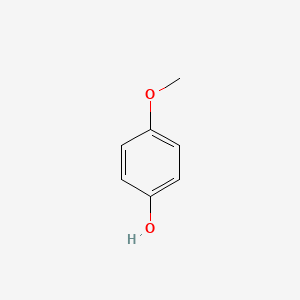 |
0.514 | D0E9CD |  |
0.298 | ||
| ENC001456 | 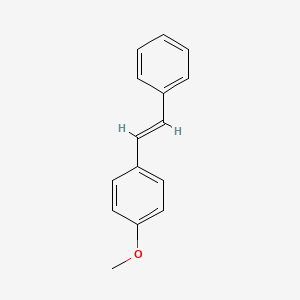 |
0.500 | D05CKR | 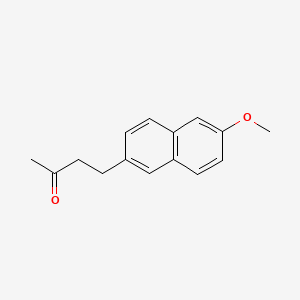 |
0.295 | ||
| ENC000201 | 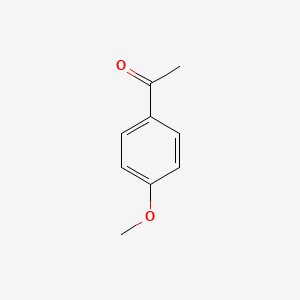 |
0.488 | D09WKB | 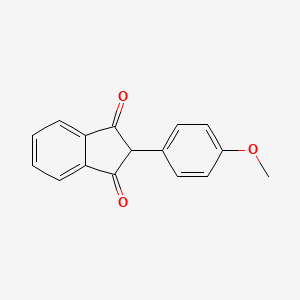 |
0.288 | ||
| ENC000310 | 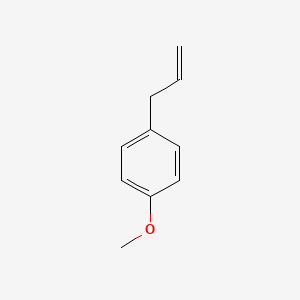 |
0.476 | D0J5DC | 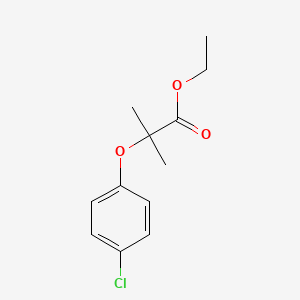 |
0.276 | ||
| ENC000223 | 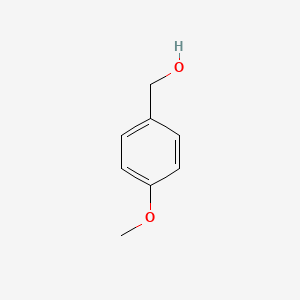 |
0.475 | D08JZS | 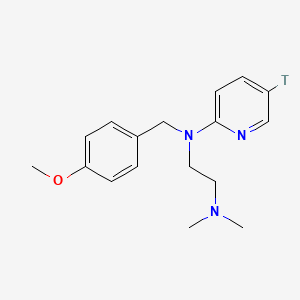 |
0.274 | ||
| ENC001461 | 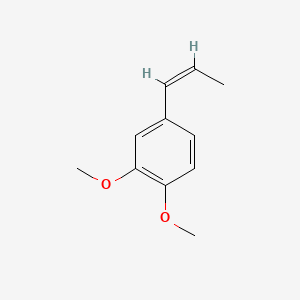 |
0.457 | D0L1WV | 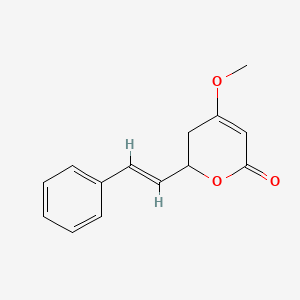 |
0.270 | ||
| ENC000298 | 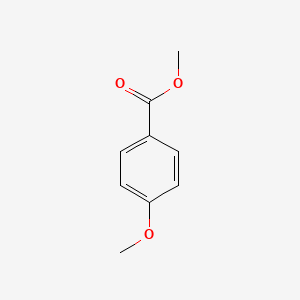 |
0.455 | D0C7AA | 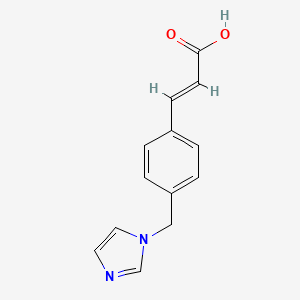 |
0.270 | ||