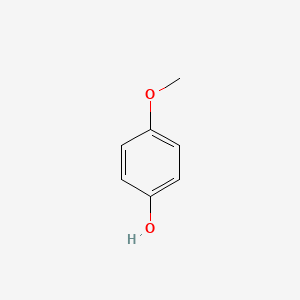
NPs Basic Information
|
Name |
4-Methoxyphenol
|
| Molecular Formula | C7H8O2 | |
| IUPAC Name* |
4-methoxyphenol
|
|
| SMILES |
COC1=CC=C(C=C1)O
|
|
| InChI |
InChI=1S/C7H8O2/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3
|
|
| InChIKey |
NWVVVBRKAWDGAB-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methoxyphenol; Mequinol; 150-76-5; 4-Hydroxyanisole; p-Hydroxyanisole; p-Methoxyphenol; Phenol, 4-methoxy-; HYDROQUINONE MONOMETHYL ETHER; Leucobasal; MEHQ; Leucodine B; Mechinolum; P-Guaiacol; Hydroquinone methyl ether; Novo-Dermoquinona; HQMME; p-Hydroxymethoxybenzene; para-methoxyphenol; 1-Hydroxy-4-methoxybenzene; Monomethyl ether hydroquinone; 4-Methoxy-phenol; PMF (antioxidant); Phenol, p-methoxy-; USAF AN-7; Mono methyl ether hydroquinone; NSC 4960; MFCD00002332; BMS 181158; BMS-181158; NSC-4960; 6HT8U7K3AM; DTXSID4020828; CHEBI:69441; NSC4960; Mequinol (INN); NCGC00091390-02; MEQUINOL [INN]; DSSTox_CID_828; DSSTox_RID_75814; DSSTox_GSID_20828; Mechinolo [DCIT]; Mequinolum; Mechinolo; Mequinolum [INN-Latin]; CAS-150-76-5; CCRIS 5531; HSDB 4258; EINECS 205-769-8; UNII-6HT8U7K3AM; Mequinol [USAN:INN:DCF]; 4methoxyphenol; paramethoxyphenol; AI3-00841; p- methoxyphenol; p-methoxy phenol; p-methoxy-phenol; 4-methoxy phenol; Eastman HQMME; 4-(methoxy)phenol; 4HA; 4KS; para- hydroxyanisole; 4-(methyloxy)phenol; HQME; MEQUINOL [HSDB]; MEQUINOL [USAN]; Mequinol (USAN/INN); Mequinol, INN, USAN; MEQUINOL [VANDF]; PHENOL,4-METHOXY; hydroxyquinone methyl ether; MEQUINOL [MART.]; hydroquinone monomethylether; CHEMBL544; MEQUINOL [WHO-DD]; EC 205-769-8; NCIMech_000709; WLN: QR DO1; SCHEMBL21009; hydroquinone mono methyl ether; MLS002454409; MEQUINOL [ORANGE BOOK]; GTPL6827; ZINC1684; P-HYDROXYANISOLE [INCI]; SOLAGE COMPONENT MEQUINOL; SCHEMBL12015251; BDBM36295; HMS2270F04; HMS3264P13; HMS3652O08; Pharmakon1600-00212037; MEQUINOL COMPONENT OF SOLAGE; 4-Methoxyphenol, analytical standard; Tox21_111125; Tox21_202367; Tox21_302876; CCG-35855; NSC760357; STL199145; AKOS000119852; Tox21_111125_1; AC-3292; AM10685; CS-W019963; DB09516; NSC-760357; PS-3375; SB40551; 4-Methoxyphenol, ReagentPlus(R), 99%; NCGC00091390-01; NCGC00091390-03; NCGC00091390-04; NCGC00256552-01; NCGC00259916-01; BP-23487; HQMME; HYDROXYQUINONE METHYL ETHER; HY-30270; NCI60_004190; SMR001252253; DB-003965; FT-0618865; M0123; S4077; SW219760-1; 4-Methoxyphenol, purum, >=98.0% (HPLC); EN300-19649; 4-Methoxyphenol, SAJ first grade, >=97.0%; D04926; P17835; AB00641905_06; AB00641905_07; A809071; SR-01000865565; Q-200491; Q2862455; SR-01000865565-2; BRD-K45216060-001-06-8; F9995-1658; Z104474598; 4-Methoxybenzyl S-(4,6-dimethylpyrimidin-2-yl)thiocarbonate
|
|
| CAS | 150-76-5 | |
| PubChem CID | 9015 | |
| ChEMBL ID | CHEMBL544 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.14 | ALogp: | 1.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.619 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.36 | MDCK Permeability: | 0.00001750 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.137 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.277 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.121 | Plasma Protein Binding (PPB): | 65.65% |
| Volume Distribution (VD): | 2.095 | Fu: | 20.55% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.865 | CYP1A2-substrate: | 0.936 |
| CYP2C19-inhibitor: | 0.64 | CYP2C19-substrate: | 0.692 |
| CYP2C9-inhibitor: | 0.113 | CYP2C9-substrate: | 0.946 |
| CYP2D6-inhibitor: | 0.466 | CYP2D6-substrate: | 0.907 |
| CYP3A4-inhibitor: | 0.099 | CYP3A4-substrate: | 0.308 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.468 | Half-life (T1/2): | 0.891 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.042 | Human Hepatotoxicity (H-HT): | 0.042 |
| Drug-inuced Liver Injury (DILI): | 0.069 | AMES Toxicity: | 0.084 |
| Rat Oral Acute Toxicity: | 0.385 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.829 | Carcinogencity: | 0.667 |
| Eye Corrosion: | 0.973 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.411 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000221 |  |
0.613 | D03UOT |  |
0.516 | ||
| ENC000223 |  |
0.606 | D0U5QK |  |
0.447 | ||
| ENC000086 | 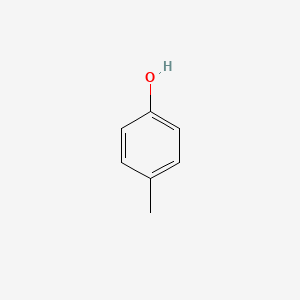 |
0.567 | D0W1RY | 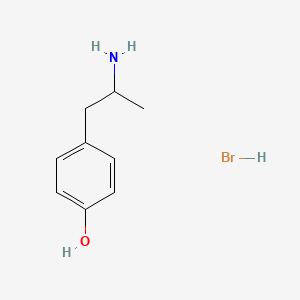 |
0.436 | ||
| ENC000740 | 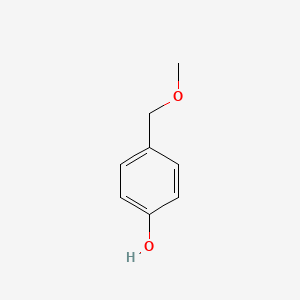 |
0.559 | D0H6TP | 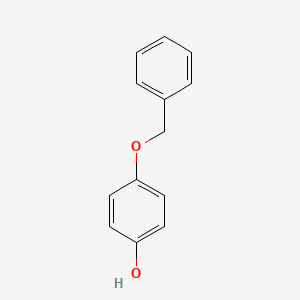 |
0.408 | ||
| ENC000195 | 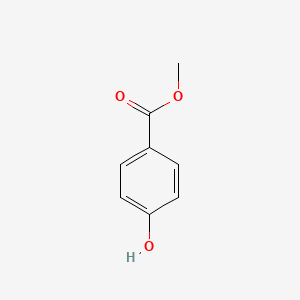 |
0.528 | D0B3QM | 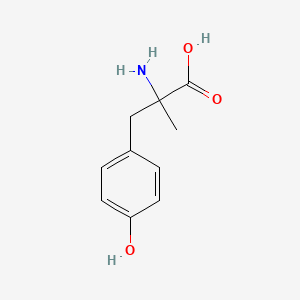 |
0.378 | ||
| ENC000201 | 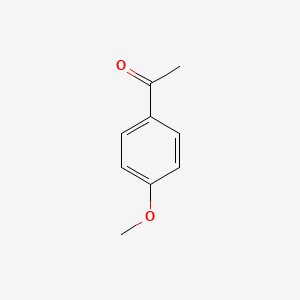 |
0.528 | D02WAB | 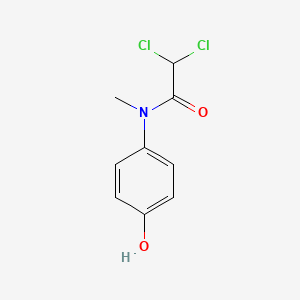 |
0.378 | ||
| ENC001021 | 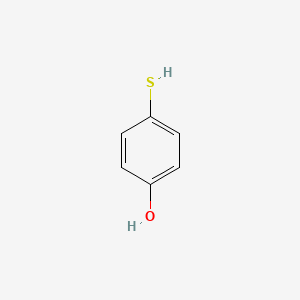 |
0.516 | D02DPU | 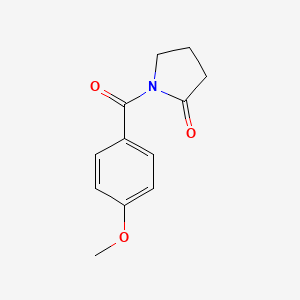 |
0.373 | ||
| ENC000310 | 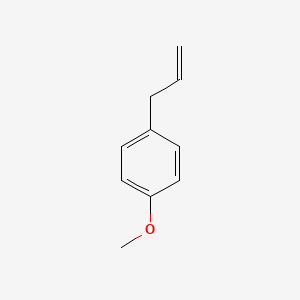 |
0.514 | D01CRB | 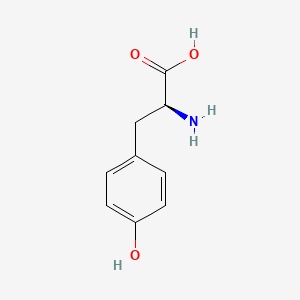 |
0.364 | ||
| ENC001460 |  |
0.514 | D0DJ1B |  |
0.333 | ||
| ENC001441 |  |
0.488 | D0P1UX |  |
0.328 | ||