NPs Basic Information
|
Name |
Vanillic Acid
|
| Molecular Formula | C8H8O4 | |
| IUPAC Name* |
4-hydroxy-3-methoxybenzoic acid
|
|
| SMILES |
COC1=C(C=CC(=C1)C(=O)O)O
|
|
| InChI |
InChI=1S/C8H8O4/c1-12-7-4-5(8(10)11)2-3-6(7)9/h2-4,9H,1H3,(H,10,11)
|
|
| InChIKey |
WKOLLVMJNQIZCI-UHFFFAOYSA-N
|
|
| Synonyms |
Vanillic acid; 4-HYDROXY-3-METHOXYBENZOIC ACID; 121-34-6; Acide vanillique; Benzoic acid, 4-hydroxy-3-methoxy-; p-Vanillic acid; 3-Methoxy-4-hydroxybenzoic acid; Vanillate; VanillicAcid; m-Anisic acid, 4-hydroxy-; Protocatechuic acid, 3-methyl ester; 4-hydroxy-3-methoxy-Benzoic acid; 4-Hydroxy-3-methoxybenzoate; NSC 3987; NSC 674322; MFCD00002551; GM8Q3JM2Y8; 4-Hydroxy-3-methoxybenzoicacid; CHEMBL120568; CHEBI:30816; VA; 4-hydroxy-3-methoxy benzoic acid; NSC-3987; NSC674322; NSC-674322; VA (VAN); VNL; 4-hydroxy-m-Anisic acid; EINECS 204-466-8; UNII-GM8Q3JM2Y8; BRN 2208364; Vanillinsaure; p-Vanillate; Vanilic acid; AI3-19542; Vanillic Acid,(S); 4-hydroxy-m-Anisate; Vanillic acid (M2); Vanillic acid, 97%; bmse000486; bmse000614; bmse010205; WLN: QVR DQ CO1; VANILLIC ACID [MI]; 3-Methoxy-4-hydroxybenzoate; SCHEMBL26179; VANILLIC ACID [INCI]; MLS000574833; 4-hydroxy-3-methoxy-Benzoate; Vanillic acid, >=97%, FG; 4-hydroxy-3methoxy benzoic acid; DTXSID6059522; FEMA NO. 3988; 4-hydroxyl-3-methoxybenzoic acid; DROXIDOPA METABOLITE (VA); NSC3987; 2-METHOXY-4-CARBOXYPHENOL; 4- hydroxy-3-methoxybenzoic acid; HMS2197E16; Protocatechuic acid 3-methyl ester; ZINC338275; HY-N0708; STR02334; BBL011982; BDBM50337364; CK2172; s5343; STL163472; AKOS000113195; CCG-266343; M-METHOXY-P-HYDROXY-BENZOIC ACID; NCGC00247610-01; AC-11841; BP-13246; SMR000156289; SY001450; DB-003804; Vanillic acid, purum, >=97.0% (HPLC); AM20050239; CS-0009728; FT-0650155; V0017; 4-HYDROXY-3-METHOXYBENZOIC ACID [FHFI]; C06672; EN300-105765; Vanillic acid, Vetec(TM) reagent grade, 97%; A804715; Q419672; Q-201921; Z381356666; Vanillic acid, certified reference material, TraceCERT(R); 3E9555E5-85F5-4FCE-A429-5182E959C6A3
|
|
| CAS | 121-34-6 | |
| PubChem CID | 8468 | |
| ChEMBL ID | CHEMBL120568 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.15 | ALogp: | 1.4 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.703 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.159 | MDCK Permeability: | 0.00000877 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.655 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.439 | Plasma Protein Binding (PPB): | 53.17% |
| Volume Distribution (VD): | 0.358 | Fu: | 36.01% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.042 | CYP1A2-substrate: | 0.69 |
| CYP2C19-inhibitor: | 0.038 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.16 |
| CYP2D6-inhibitor: | 0.015 | CYP2D6-substrate: | 0.13 |
| CYP3A4-inhibitor: | 0.039 | CYP3A4-substrate: | 0.07 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.899 | Half-life (T1/2): | 0.941 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.224 |
| Drug-inuced Liver Injury (DILI): | 0.857 | AMES Toxicity: | 0.015 |
| Rat Oral Acute Toxicity: | 0.053 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.154 | Carcinogencity: | 0.062 |
| Eye Corrosion: | 0.202 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.12 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
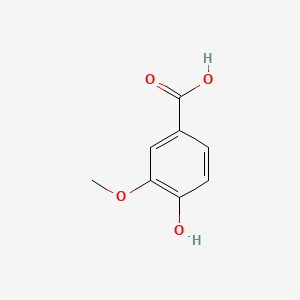
| ENC001056 | 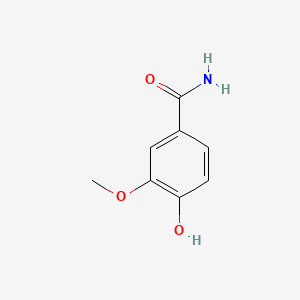 |
0.730 | D0C4YC | 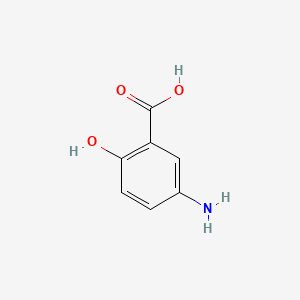 |
0.452 | ||
| ENC000777 | 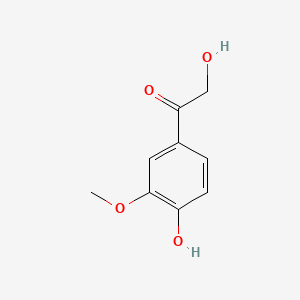 |
0.718 | D0E9CD |  |
0.442 | ||
| ENC000002 | 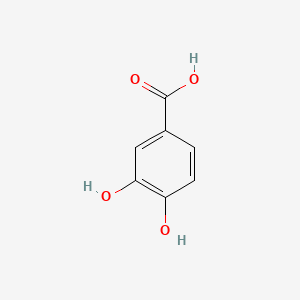 |
0.605 | D01WJL | 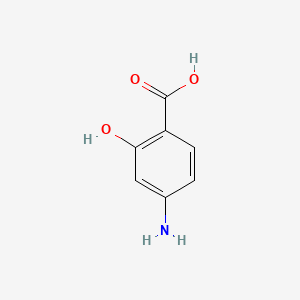 |
0.419 | ||
| ENC001101 | 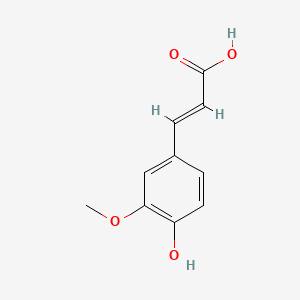 |
0.591 | D0U0OT | 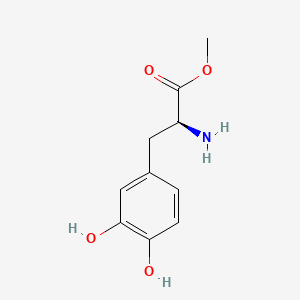 |
0.385 | ||
| ENC000325 | 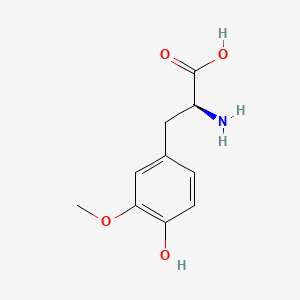 |
0.565 | D07HBX |  |
0.372 | ||
| ENC001055 | 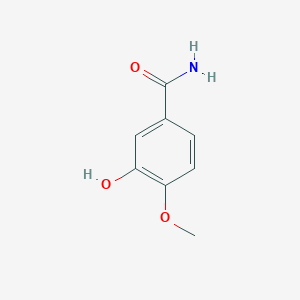 |
0.561 | D0V9EN |  |
0.367 | ||
| ENC000172 | 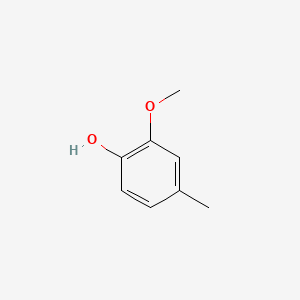 |
0.553 | D0BA6T | 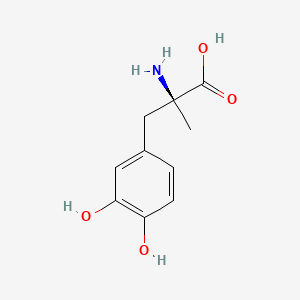 |
0.365 | ||
| ENC000068 | 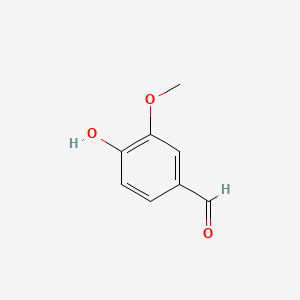 |
0.550 | D08HVR | 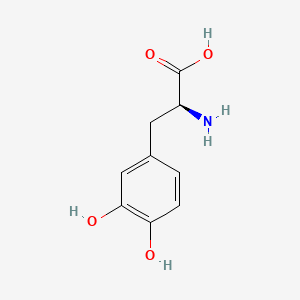 |
0.353 | ||
| ENC000027 | 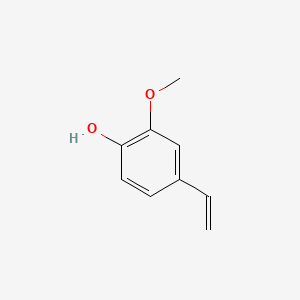 |
0.512 | D03LGG | 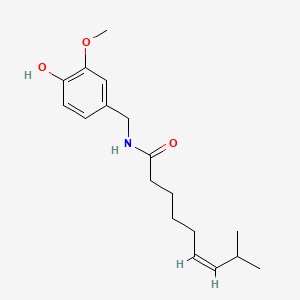 |
0.348 | ||
| ENC000507 | 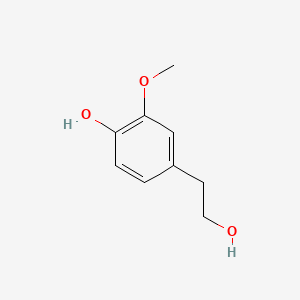 |
0.512 | D0U5CE | 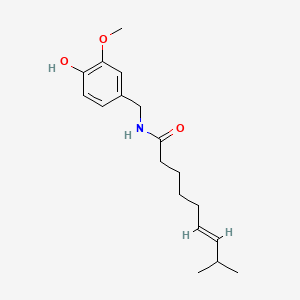 |
0.348 | ||