NPs Basic Information
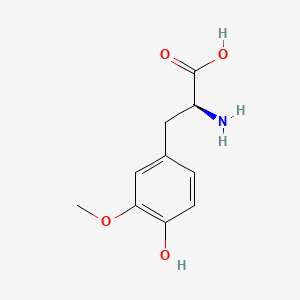
|
Name |
3-Methoxy-L-tyrosine
|
| Molecular Formula | C10H13NO4 | |
| IUPAC Name* |
(2S)-2-amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid
|
|
| SMILES |
COC1=C(C=CC(=C1)C[C@@H](C(=O)O)N)O
|
|
| InChI |
InChI=1S/C10H13NO4/c1-15-9-5-6(2-3-8(9)12)4-7(11)10(13)14/h2-3,5,7,12H,4,11H2,1H3,(H,13,14)/t7-/m0/s1
|
|
| InChIKey |
PFDUUKDQEHURQC-ZETCQYMHSA-N
|
|
| Synonyms |
3-METHOXY-L-TYROSINE; 3-O-METHYLDOPA; 300-48-1; L-Tyrosine, 3-methoxy-; (2S)-2-amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid; 3-O-Methyl Dopa; L-3-O-Methyl-DOPA; V3O7J20DWN; CHEBI:82913; (S)-2-Amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid; L-3-methoxy-4-hydroxyphenylalanine; L-4-Hydroxy-3-methoxyphenylalanine; 3-O-Methyldopa, L-; UNII-V3O7J20DWN; 3-methoxy-4-hydroxyphenylalanine; OM-DOPA; 3-(4-hydroxy-3-methoxyphenyl)-L-alanine; L-3-(4-hydroxy-3-methoxyphenyl)-alanine; 3-O-Methyl-L-DOPA; 3-O-Methyl-a-methyldopa; L-3-MTO; SCHEMBL180104; 3-O-METHYLDOPA [MI]; (-)-3-O-METHYLDOPA; CHEMBL1314652; ZINC85742; DTXSID401315157; MFCD07784093; AKOS012010388; HY-113468A; NCGC00163350-01; 3-Methoxy-L-tyrosine monohydrate, powder; AS-57781; DB-016869; AM20041134; CS-0131977; D95468; EN300-321078; Q15410220; (2S)-2-amino-3-(4-hydroxy-3-methoxy-phenyl)propionic acid; 3YM
|
|
| CAS | 300-48-1 | |
| PubChem CID | 9307 | |
| ChEMBL ID | CHEMBL1314652 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 211.21 | ALogp: | -2.4 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 92.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.683 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.709 | MDCK Permeability: | 0.00178358 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.9 |
| Human Intestinal Absorption (HIA): | 0.017 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.355 | Plasma Protein Binding (PPB): | 25.85% |
| Volume Distribution (VD): | 0.487 | Fu: | 73.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.034 | CYP1A2-substrate: | 0.097 |
| CYP2C19-inhibitor: | 0.054 | CYP2C19-substrate: | 0.063 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.439 |
| CYP2D6-inhibitor: | 0.027 | CYP2D6-substrate: | 0.35 |
| CYP3A4-inhibitor: | 0.028 | CYP3A4-substrate: | 0.057 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 16.566 | Half-life (T1/2): | 0.885 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.047 | Human Hepatotoxicity (H-HT): | 0.237 |
| Drug-inuced Liver Injury (DILI): | 0.019 | AMES Toxicity: | 0.033 |
| Rat Oral Acute Toxicity: | 0.475 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.214 | Carcinogencity: | 0.132 |
| Eye Corrosion: | 0.008 | Eye Irritation: | 0.049 |
| Respiratory Toxicity: | 0.379 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000127 | 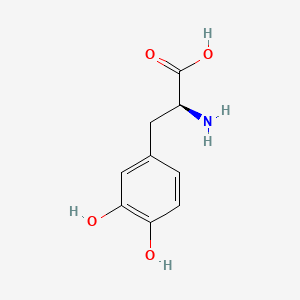 |
0.674 | D08HVR | 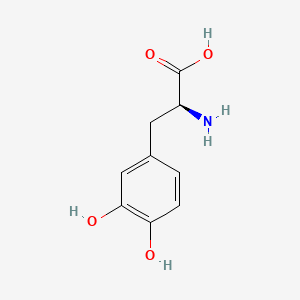 |
0.674 | ||
| ENC000296 | 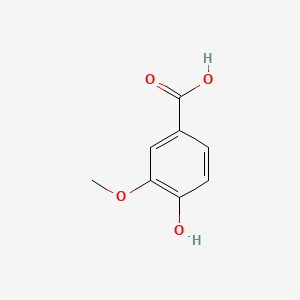 |
0.565 | D0U0OT | 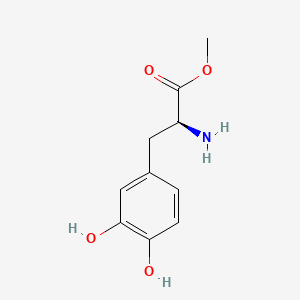 |
0.600 | ||
| ENC000507 | 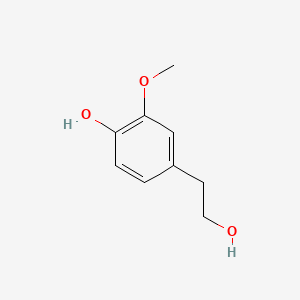 |
0.553 | D01CRB | 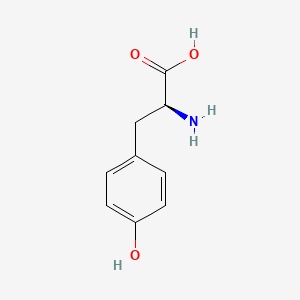 |
0.531 | ||
| ENC001056 | 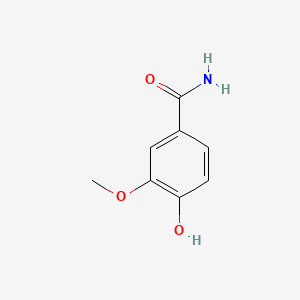 |
0.532 | D0R1CR |  |
0.431 | ||
| ENC000129 | 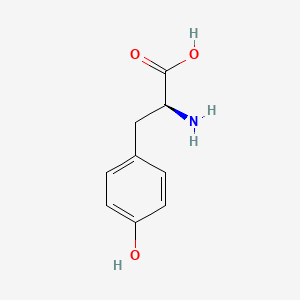 |
0.531 | D0S6JG | 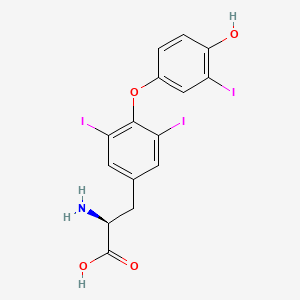 |
0.417 | ||
| ENC000777 | 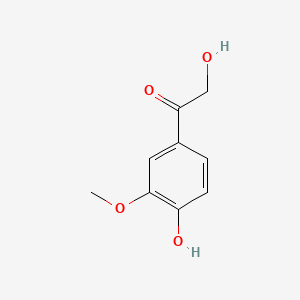 |
0.531 | D0BA6T | 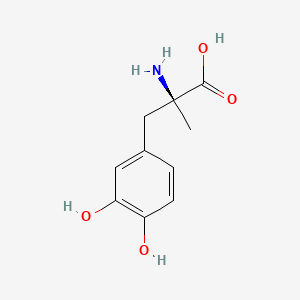 |
0.411 | ||
| ENC000095 | 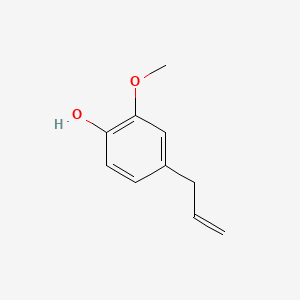 |
0.521 | D0U5CE | 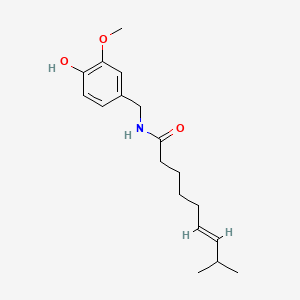 |
0.403 | ||
| ENC001101 | 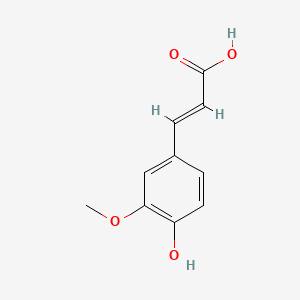 |
0.500 | D03LGG | 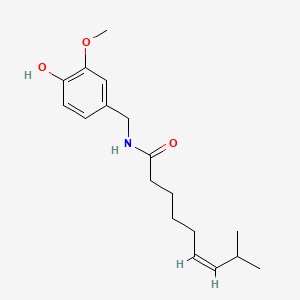 |
0.403 | ||
| ENC000068 | 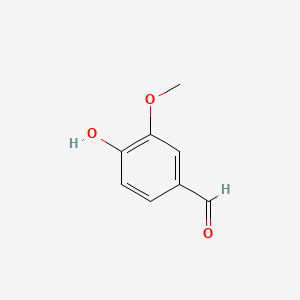 |
0.458 | D0P7JZ | 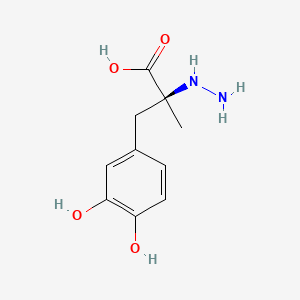 |
0.390 | ||
| ENC000172 | 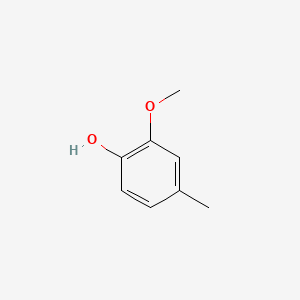 |
0.457 | D0I3RO | 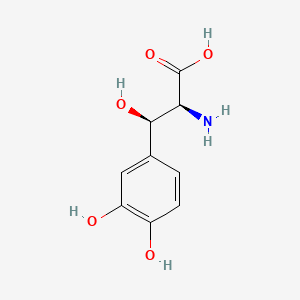 |
0.386 | ||