NPs Basic Information
|
Name |
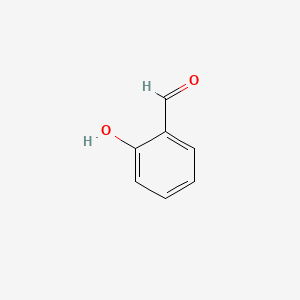
Salicylaldehyde
|
| Molecular Formula | C7H6O2 | |
| IUPAC Name* |
2-hydroxybenzaldehyde
|
|
| SMILES |
C1=CC=C(C(=C1)C=O)O
|
|
| InChI |
InChI=1S/C7H6O2/c8-5-6-3-1-2-4-7(6)9/h1-5,9H
|
|
| InChIKey |
SMQUZDBALVYZAC-UHFFFAOYSA-N
|
|
| Synonyms |
SALICYLALDEHYDE; 2-Hydroxybenzaldehyde; 90-02-8; o-Hydroxybenzaldehyde; o-Formylphenol; Salicylal; 2-Formylphenol; Benzaldehyde, 2-hydroxy-; Salicylic aldehyde; Salicyladehyde; Benzaldehyde, o-hydroxy-; Salicylaldehyd; Salizylaldehyd; 2-HYDROXY-BENZALDEHYDE; FEMA No. 3004; salicyl aldehyde; MFCD00003317; NSC 49178; 2-hydroxy benzaldehyde; CHEMBL108925; CHEBI:16008; 17K64GZH20; NSC-49178; Benzaldehyde, hydroxy-; DSSTox_CID_1792; DSSTox_RID_76329; DSSTox_GSID_21792; CAS-90-02-8; CCRIS 7451; HSDB 721; NSC-83559; NSC-83560; NSC-83561; NSC-83562; NSC-97202; NSC-112278; EINECS 201-961-0; BRN 0471388; UNII-17K64GZH20; salicylylaldehyde; AI3-02174; 28777-87-9; hydroxylbenzaldehyde; hydroxy benzaldehyde; hydroxyl benzaldehyde; 2-hyroxy-benzaldehyde; 2-oxidanylbenzaldehyde; o-hydroxy benzaldehyde; Salicylaldehyde, 8CI; 2- hydroxybenzaldehyde; WLN: VHR BQ; bmse000677; EC 201-961-0; SALICYLALDEHYDE [MI]; SCHEMBL15395; SALICYLALDEHYDE [FCC]; 4-08-00-00176 (Beilstein Handbook Reference); SALICYLALDEHYDE [FHFI]; SALICYLALDEHYDE [HSDB]; Salicylaldehyde, >=98%, FG; DTXSID1021792; FEMA 3004; Salicylaldehyde, p.a., 99.0%; ZINC896073; BCP31374; CS-D1188; NSC49178; Salicylaldehyde, analytical standard; Tox21_201737; Tox21_302929; BDBM50139367; NSC187662; Salicylaldehyde, reagent grade, 98%; STL194289; AKOS000119187; 2-hydroxybenzaldehyde (salicylaldehyde); NSC-187662; NCGC00249108-01; NCGC00256460-01; NCGC00259286-01; AS-13997; FT-0648915; Salicylaldehyde, redist., >=99.0% (GC); EN300-18033; 90S028; C06202; H-3700; A843413; Q414492; Z57127523; F2190-0607; 2-Hydroxybenzaldehyde;o-Hydroxybenzaldehyde;o-Formylphenol; 27761-48-4
|
|
| CAS | 90-02-8 | |
| PubChem CID | 6998 | |
| ChEMBL ID | CHEMBL108925 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 122.12 | ALogp: | 1.8 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.575 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.384 | MDCK Permeability: | 0.00001540 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.014 | 20% Bioavailability (F20%): | 0.284 |
| 30% Bioavailability (F30%): | 0.524 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.517 | Plasma Protein Binding (PPB): | 74.64% |
| Volume Distribution (VD): | 0.896 | Fu: | 22.98% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.744 | CYP1A2-substrate: | 0.123 |
| CYP2C19-inhibitor: | 0.152 | CYP2C19-substrate: | 0.151 |
| CYP2C9-inhibitor: | 0.073 | CYP2C9-substrate: | 0.788 |
| CYP2D6-inhibitor: | 0.039 | CYP2D6-substrate: | 0.507 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.22 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.141 | Half-life (T1/2): | 0.853 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.021 |
| Drug-inuced Liver Injury (DILI): | 0.039 | AMES Toxicity: | 0.271 |
| Rat Oral Acute Toxicity: | 0.033 | Maximum Recommended Daily Dose: | 0.038 |
| Skin Sensitization: | 0.706 | Carcinogencity: | 0.357 |
| Eye Corrosion: | 0.983 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.965 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001547 | 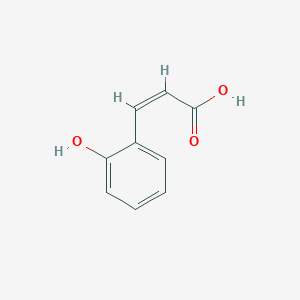 |
0.568 | D07HBX |  |
0.486 | ||
| ENC000021 | 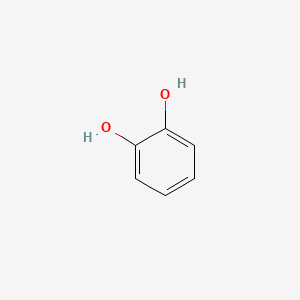 |
0.516 | D0E9CD |  |
0.341 | ||
| ENC000028 | 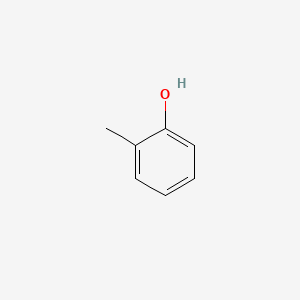 |
0.516 | D0F5ZM | 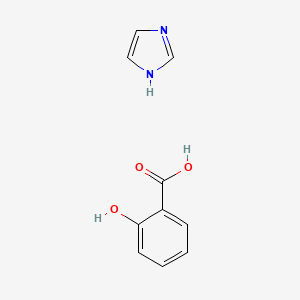 |
0.340 | ||
| ENC000696 | 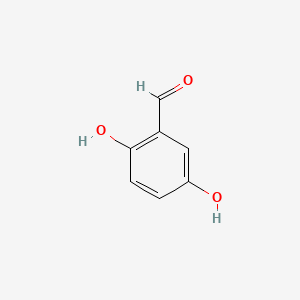 |
0.486 | D01ZJK |  |
0.333 | ||
| ENC000108 | 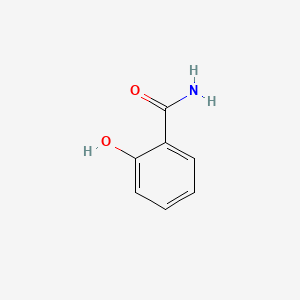 |
0.486 | D05OIS |  |
0.333 | ||
| ENC000033 | 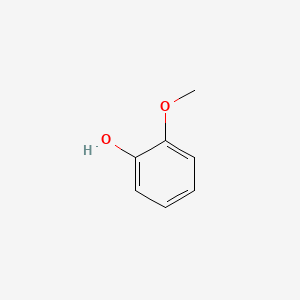 |
0.471 | D0X9RY |  |
0.316 | ||
| ENC000341 |  |
0.462 | D0GY5Z | 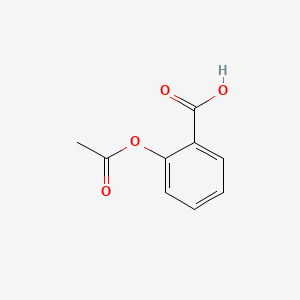 |
0.304 | ||
| ENC000012 | 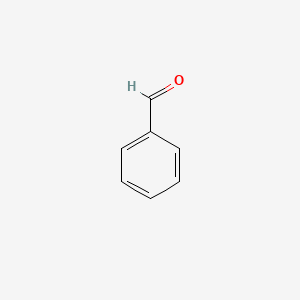 |
0.455 | D0N3UL | 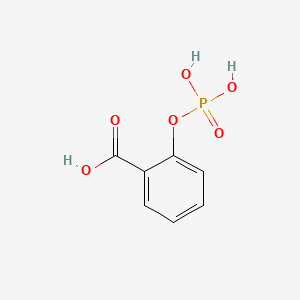 |
0.292 | ||
| ENC000409 | 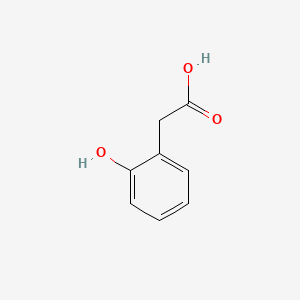 |
0.447 | D0R1CR |  |
0.289 | ||
| ENC000104 | 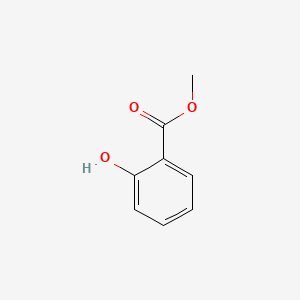 |
0.447 | D0L5PO | 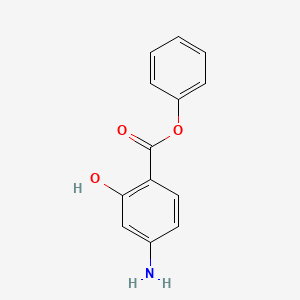 |
0.281 | ||