NPs Basic Information
|
Name |
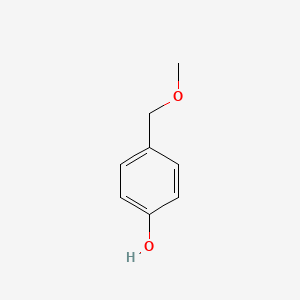
4-(Methoxymethyl)phenol
|
| Molecular Formula | C8H10O2 | |
| IUPAC Name* |
4-(methoxymethyl)phenol
|
|
| SMILES |
COCC1=CC=C(C=C1)O
|
|
| InChI |
InChI=1S/C8H10O2/c1-10-6-7-2-4-8(9)5-3-7/h2-5,9H,6H2,1H3
|
|
| InChIKey |
AHXXIALEMINDAW-UHFFFAOYSA-N
|
|
| Synonyms |
4-(Methoxymethyl)phenol; 5355-17-9; Phenol, 4-(methoxymethyl)-; 4-Methoxymethylphenol; alpha-Methoxy-p-cresol; 4-Hydroxybenzyl methyl ether; 4-Methoxymethyl-phenol; p-(methoxymethyl)phenol; p-hydroxybenzyl methyl ether; 17CJ5983VN; MFCD00020186; UNII-17CJ5983VN; EINECS 226-334-9; 4-(methoxymethyl)-phenol; SCHEMBL63724; CHEMBL3929528; DTXSID2074746; .ALPHA.-METHOXY-P-CRESOL; CHEBI:133885; ZINC394208; P-CRESOL, .ALPHA.-METHOXY; AKOS006273184; DS-14089; SY026430; DB-071699; FT-0717554; A25732; EN300-1851052; 020M186; Q27251899; ETHER,(4-HYDROXYBENZYL),METHYL (TOLUENE,4-HYDROXY,ALPHA-METHOXY)
|
|
| CAS | 5355-17-9 | |
| PubChem CID | 79310 | |
| ChEMBL ID | CHEMBL3929528 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.16 | ALogp: | 0.8 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.678 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.273 | MDCK Permeability: | 0.00002590 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.087 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.014 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.12 | Plasma Protein Binding (PPB): | 35.76% |
| Volume Distribution (VD): | 1.983 | Fu: | 53.84% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.426 | CYP1A2-substrate: | 0.708 |
| CYP2C19-inhibitor: | 0.269 | CYP2C19-substrate: | 0.509 |
| CYP2C9-inhibitor: | 0.056 | CYP2C9-substrate: | 0.245 |
| CYP2D6-inhibitor: | 0.206 | CYP2D6-substrate: | 0.765 |
| CYP3A4-inhibitor: | 0.037 | CYP3A4-substrate: | 0.417 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.978 | Half-life (T1/2): | 0.866 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.054 | Human Hepatotoxicity (H-HT): | 0.038 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.203 |
| Rat Oral Acute Toxicity: | 0.775 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.855 | Carcinogencity: | 0.685 |
| Eye Corrosion: | 0.436 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.047 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004860 | 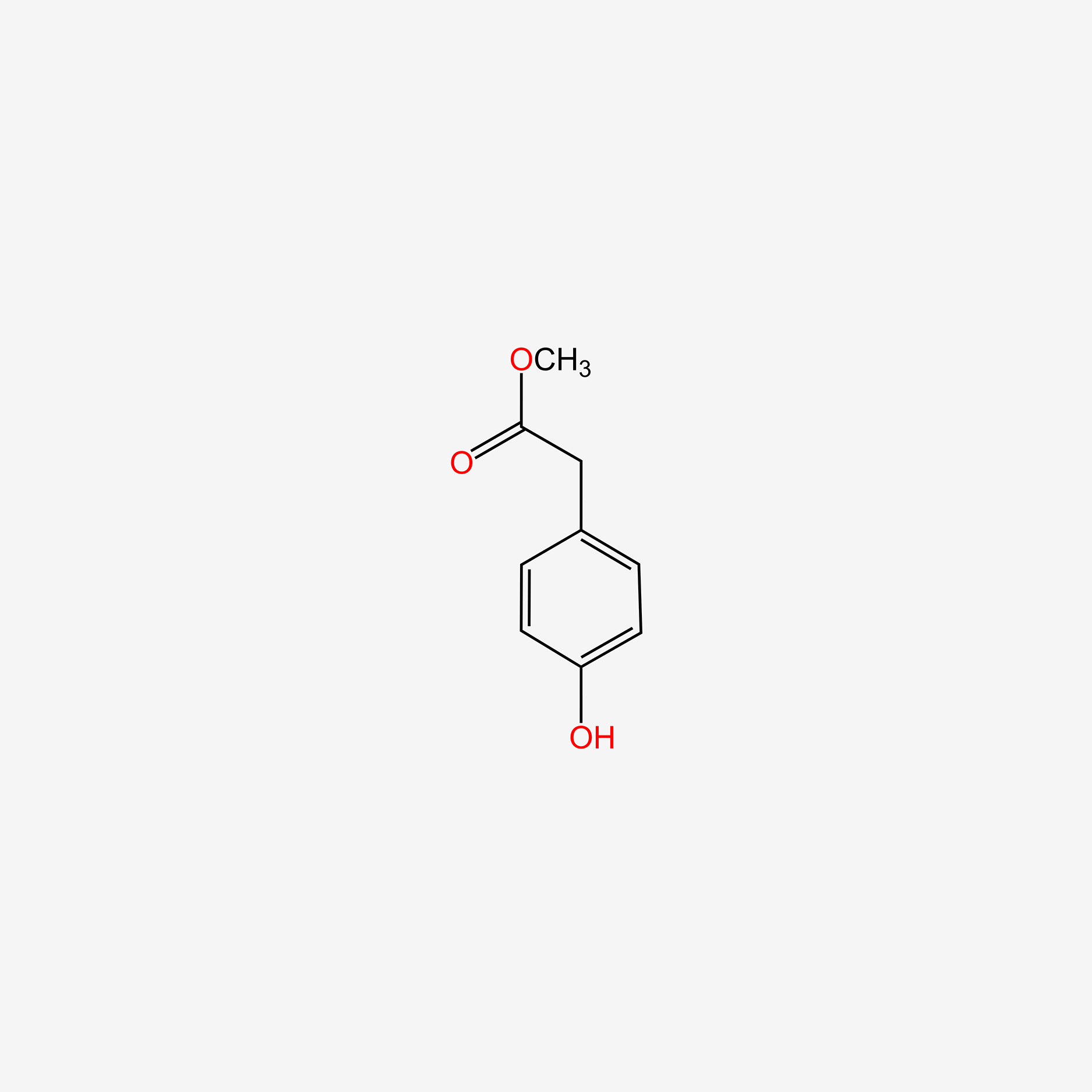 |
0.605 | D0W1RY | 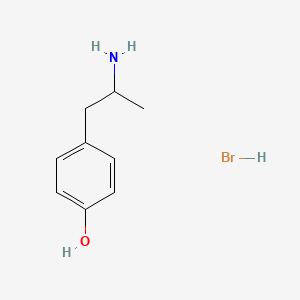 |
0.553 | ||
| ENC000318 | 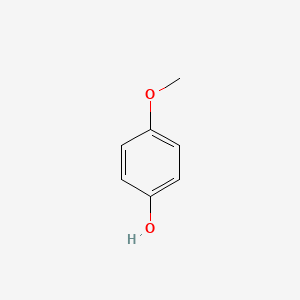 |
0.559 | D0B3QM | 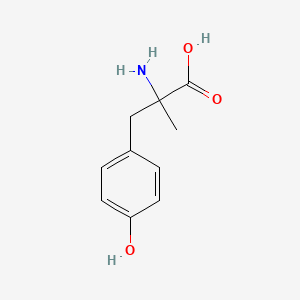 |
0.477 | ||
| ENC000350 | 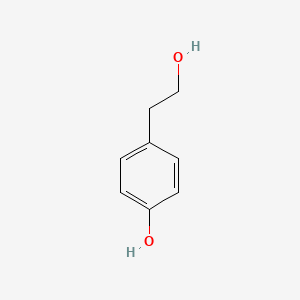 |
0.556 | D03UOT |  |
0.471 | ||
| ENC000676 | 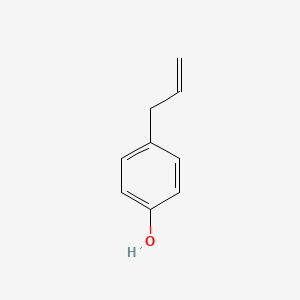 |
0.556 | D01CRB | 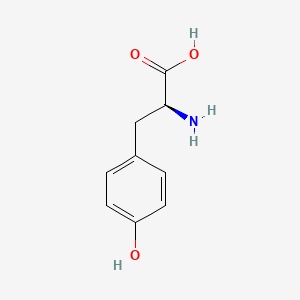 |
0.465 | ||
| ENC000006 | 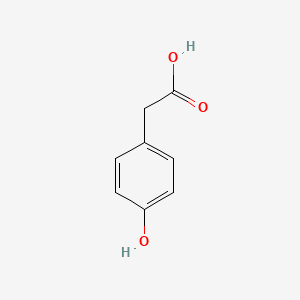 |
0.526 | D0U5QK | 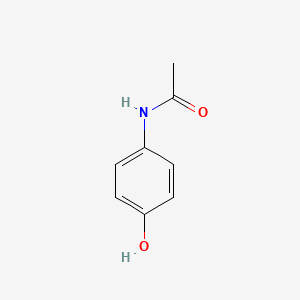 |
0.415 | ||
| ENC000774 | 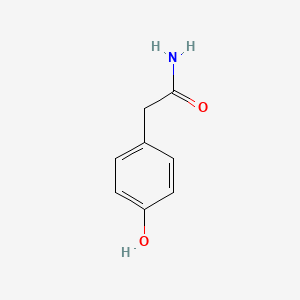 |
0.526 | D0H6TP | 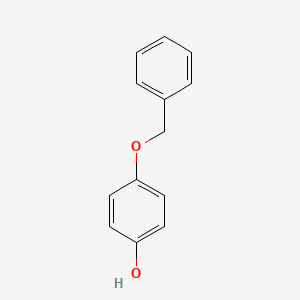 |
0.412 | ||
| ENC001422 | 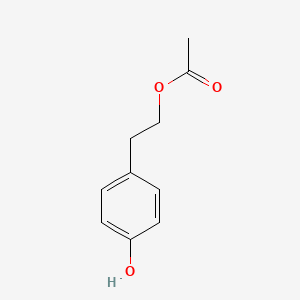 |
0.524 | D0S2BV | 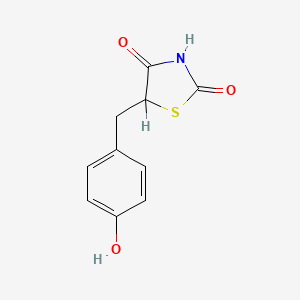 |
0.400 | ||
| ENC000086 | 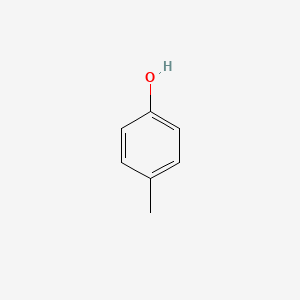 |
0.515 | D02WAB | 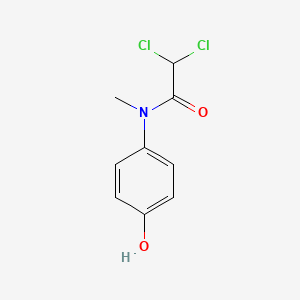 |
0.354 | ||
| ENC000870 | 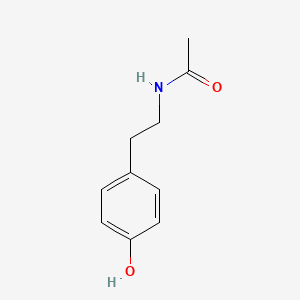 |
0.488 | D06CDO | 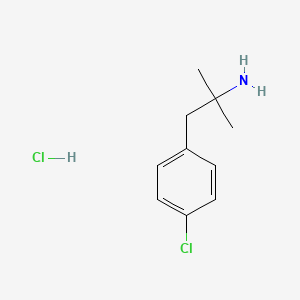 |
0.326 | ||
| ENC000195 | 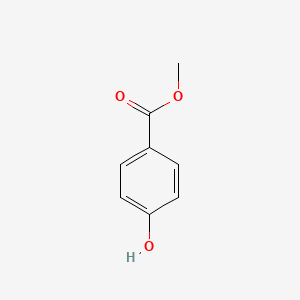 |
0.487 | D02AQY | 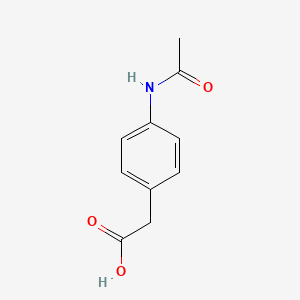 |
0.320 | ||