NPs Basic Information
|
Name |
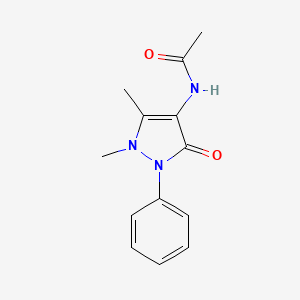
4-Acetamidoantipyrine
|
| Molecular Formula | C13H15N3O2 | |
| IUPAC Name* |
N-(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)acetamide
|
|
| SMILES |
CC1=C(C(=O)N(N1C)C2=CC=CC=C2)NC(=O)C
|
|
| InChI |
InChI=1S/C13H15N3O2/c1-9-12(14-10(2)17)13(18)16(15(9)3)11-7-5-4-6-8-11/h4-8H,1-3H3,(H,14,17)
|
|
| InChIKey |
OIAGWXKSCXPNNZ-UHFFFAOYSA-N
|
|
| Synonyms |
4-Acetamidoantipyrine; 83-15-8; 4-Acetaminoantipyrine; 4-Acetylaminophenazone; 4-Acetylaminoantipyrine; Acetamidoantipyrine; Acetylaminoantipyrine; 4-Acetoaminoantipyrine; 4-Acetamido Antipyrine; Antipyrine, 4-acetamido-; Acetamide, N-antipyrinyl-; N-Acetyl-4-aminoantipyrine; N-(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)acetamide; Acetamide, N-(antipyrinyl)-; N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)acetamide; Acetyl-4-aminoantipyrine; NSC 331807; Acetamide, N-(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)-; CHEBI:83513; 535H9N144Z; NSC-331807; 4-Acetamidoantipyrin; N-Acetylaminoantipyrine; UNII-535H9N144Z; Antipyrine Impurity; EINECS 201-457-0; N-Antipyrinylacetamide; N-(2,3-Dimethyl-5-oxo-1-phenyl-3-pyrazolin-4-yl)acetamide; AI3-52432; Aminoantipyrine, N-acetyl-; 4-Methylamino antipyrine-d3; 4-(N-Acetylamino)antipyrine; Oprea1_016919; Oprea1_507768; F0034-0117; SCHEMBL5050992; CHEMBL1831257; OIAGWXKSCXPNNZ-UHFFFAOYSA-; ZINC41021; DTXSID40232106; AAA METABOLITE OF METAMIZOLE; MFCD00003141; NSC331807; AKOS000746331; SDCCGMLS-0064808.P001; SDCCGMLS-0064808.P002; 4-Acetamidoantipyrine, analytical standard; J47.302B; MS-10581; DB-056685; CS-0313868; FT-0631379; A840513; SR-01000395595; SR-01000395595-1; 4-Acetylaminoantipyrine 10 microg/mL in Acetonitrile; Q27156901; 4-ACETAMIDO-2,3-DIMETHYL-1-PHENYLPYRAZOL-5-ONE; N-(1,5-dimethyl-3-oxo-2-phenyl-pyrazol-4-yl)acetamide; Acetamide,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)-; Acetamide,N-(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)-; N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)acetamide #; N-(2,3-Dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)acetamide
|
|
| CAS | 83-15-8 | |
| PubChem CID | 65743 | |
| ChEMBL ID | CHEMBL1831257 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 245.28 | ALogp: | 0.0 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 52.6 | Aromatic Rings: | 2 |
| Heavy Atoms: | 18 | QED Weighted: | 0.879 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.554 | MDCK Permeability: | 0.00001330 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.765 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.044 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.974 | Plasma Protein Binding (PPB): | 15.72% |
| Volume Distribution (VD): | 1.546 | Fu: | 71.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.046 | CYP1A2-substrate: | 0.571 |
| CYP2C19-inhibitor: | 0.031 | CYP2C19-substrate: | 0.894 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.522 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.471 |
| CYP3A4-inhibitor: | 0.013 | CYP3A4-substrate: | 0.847 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.981 | Half-life (T1/2): | 0.546 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.577 |
| Drug-inuced Liver Injury (DILI): | 0.946 | AMES Toxicity: | 0.186 |
| Rat Oral Acute Toxicity: | 0.08 | Maximum Recommended Daily Dose: | 0.03 |
| Skin Sensitization: | 0.26 | Carcinogencity: | 0.104 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.028 |
| Respiratory Toxicity: | 0.256 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000391 | 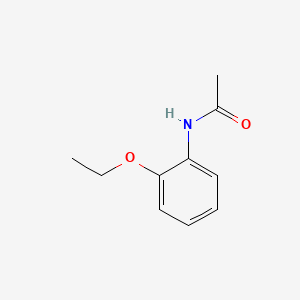 |
0.349 | D06IXT | 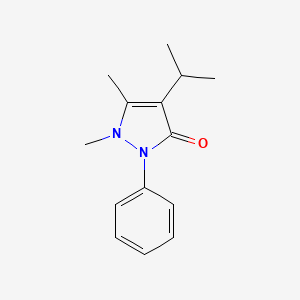 |
0.610 | ||
| ENC000192 |  |
0.345 | D06DLI | 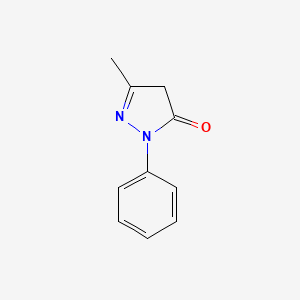 |
0.387 | ||
| ENC000370 | 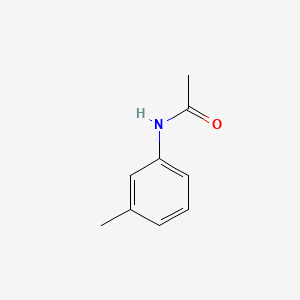 |
0.339 | D02WCI | 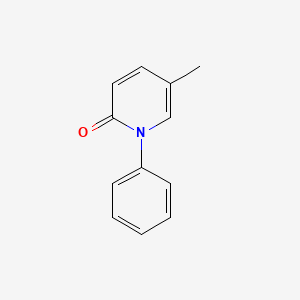 |
0.369 | ||
| ENC000693 | 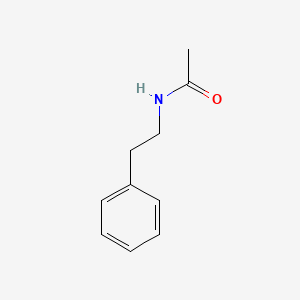 |
0.339 | D08EOD | 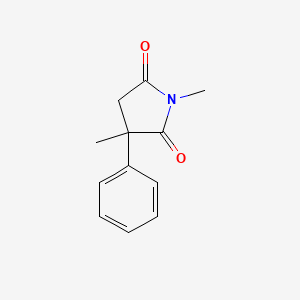 |
0.343 | ||
| ENC002213 | 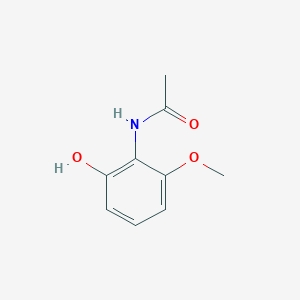 |
0.333 | D06BYV | 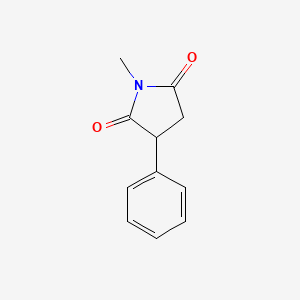 |
0.333 | ||
| ENC001033 | 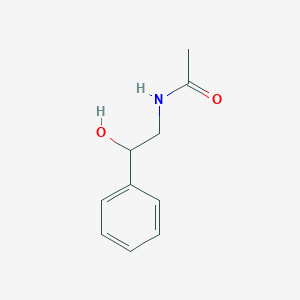 |
0.328 | D0B1FE | 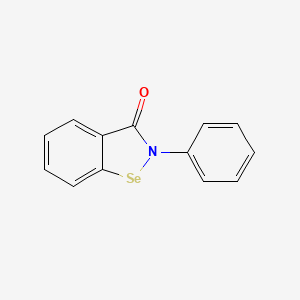 |
0.333 | ||
| ENC000218 | 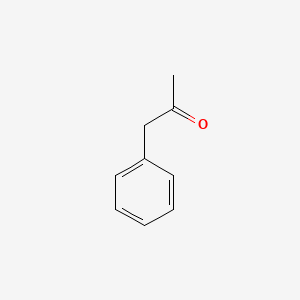 |
0.328 | D07RGW | 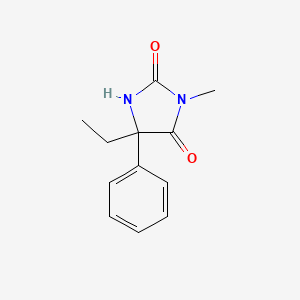 |
0.329 | ||
| ENC000717 | 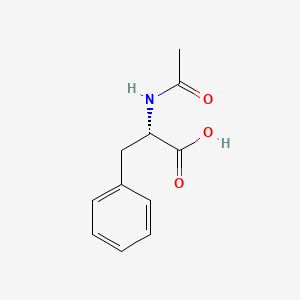 |
0.324 | D08UMH | 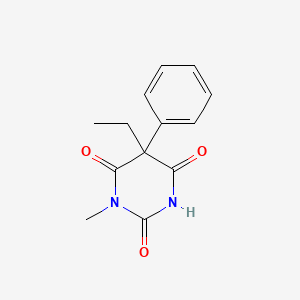 |
0.324 | ||
| ENC001042 | 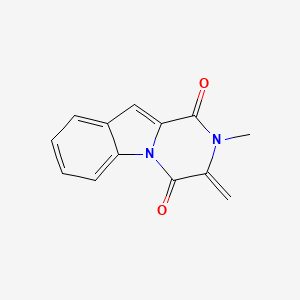 |
0.315 | D03GET | 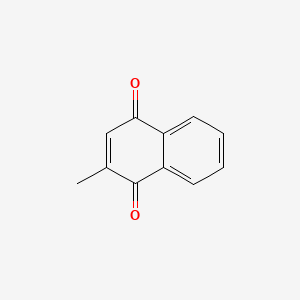 |
0.308 | ||
| ENC001970 | 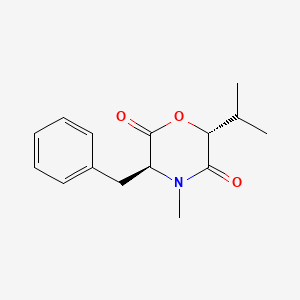 |
0.312 | D03KOZ | 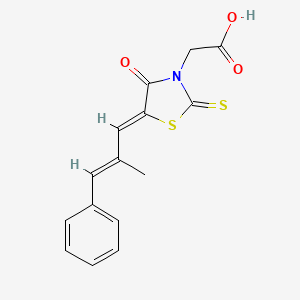 |
0.305 | ||