NPs Basic Information
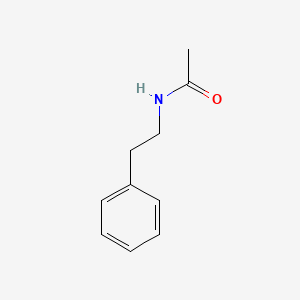
|
Name |
N-(2-Phenylethyl)acetamide
|
| Molecular Formula | C10H13NO | |
| IUPAC Name* |
N-(2-phenylethyl)acetamide
|
|
| SMILES |
CC(=O)NCCC1=CC=CC=C1
|
|
| InChI |
InChI=1S/C10H13NO/c1-9(12)11-8-7-10-5-3-2-4-6-10/h2-6H,7-8H2,1H3,(H,11,12)
|
|
| InChIKey |
MODKMHXGCGKTLE-UHFFFAOYSA-N
|
|
| Synonyms |
N-Phenethylacetamide; N-(2-Phenylethyl)acetamide; 877-95-2; N-Acetyl-2-phenylethylamine; N-Acetylphenethylamine; Acetamide, N-(2-phenylethyl)-; N-(Phenethyl)acetamide; N-Acetylphenylethylamine; Acetamide, N-phenethyl-; N-beta-Phenylethylacetamide; N-phenethyl-acetamide; N-(2-phenylethyl)-acetamide; N-2-Phenethylacetamide; N-(2-Phenethyl)acetamide; 2JXY218SZI; CHEBI:18177; NSC-7177; NSC 7177; EINECS 212-897-8; UNII-2JXY218SZI; BRN 2208721; Acetamide,N-(2-phenylethyl)-; acetylphenethylamine; 54W; Glipizide Impurity 1; N-Acetyl-phenethylamine; (2-Phenethyl)acetamide; N-(2-phenethyl)-acetamide; SCHEMBL7858; N-(ss-Phenylethyl)acetamide; CHEMBL99827; N-acetyl (2-phenyl)ethylamine; F0020-1761; MEGxm0_000495; DTXSID50236574; n-(2-phenylethyl)acetamide (en); NSC7177; ZINC163574; MFCD00026177; STK364324; AKOS003082304; GS-0407; N-(.BETA.-PHENYLETHYL)ACETAMIDE; NCGC00332267-01; DB-026826; BB 0263213; FT-0629790; P1066; C06746; AB00172777-03; NCGC00332267-02!N-(2-phenylethyl)acetamide; A862412; AS-871/40170832; Q27102876; Z27761567
|
|
| CAS | 877-95-2 | |
| PubChem CID | 70143 | |
| ChEMBL ID | CHEMBL99827 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 163.22 | ALogp: | 1.5 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.724 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.351 | MDCK Permeability: | 0.00004390 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.143 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.592 |
| 30% Bioavailability (F30%): | 0.874 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.834 | Plasma Protein Binding (PPB): | 34.20% |
| Volume Distribution (VD): | 1.184 | Fu: | 45.30% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.752 | CYP1A2-substrate: | 0.498 |
| CYP2C19-inhibitor: | 0.435 | CYP2C19-substrate: | 0.797 |
| CYP2C9-inhibitor: | 0.047 | CYP2C9-substrate: | 0.126 |
| CYP2D6-inhibitor: | 0.235 | CYP2D6-substrate: | 0.462 |
| CYP3A4-inhibitor: | 0.043 | CYP3A4-substrate: | 0.359 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.861 | Half-life (T1/2): | 0.802 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.038 | Human Hepatotoxicity (H-HT): | 0.32 |
| Drug-inuced Liver Injury (DILI): | 0.181 | AMES Toxicity: | 0.194 |
| Rat Oral Acute Toxicity: | 0.016 | Maximum Recommended Daily Dose: | 0.074 |
| Skin Sensitization: | 0.263 | Carcinogencity: | 0.064 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.18 |
| Respiratory Toxicity: | 0.021 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000216 | 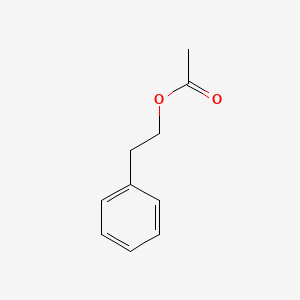 |
0.619 | D0P9AC | 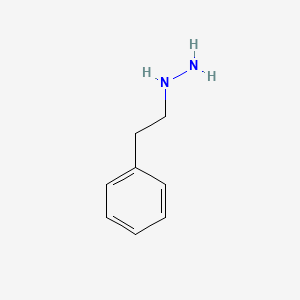 |
0.615 | ||
| ENC000218 | 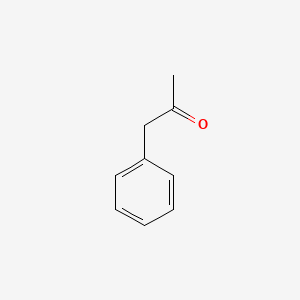 |
0.590 | D0P2GK | 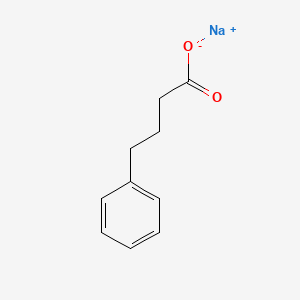 |
0.533 | ||
| ENC000004 | 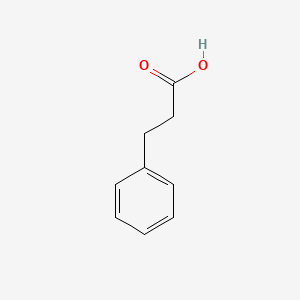 |
0.585 | D00DZN |  |
0.511 | ||
| ENC000779 | 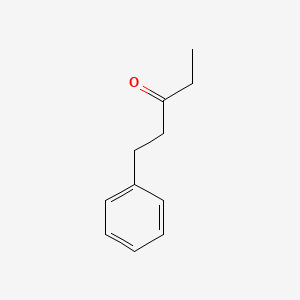 |
0.581 | D05OFX | 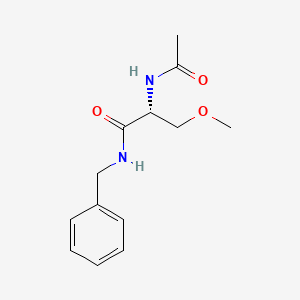 |
0.474 | ||
| ENC000217 | 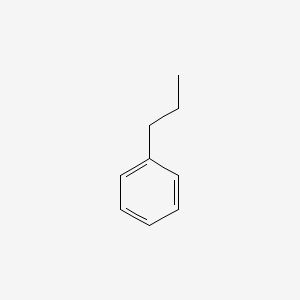 |
0.579 | D05OIS |  |
0.462 | ||
| ENC000598 | 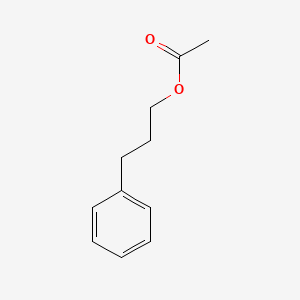 |
0.578 | D07ONP | 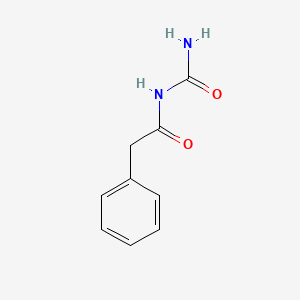 |
0.458 | ||
| ENC000870 | 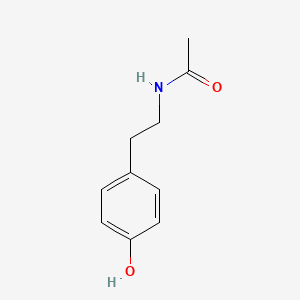 |
0.556 | D0R1CR |  |
0.457 | ||
| ENC000308 | 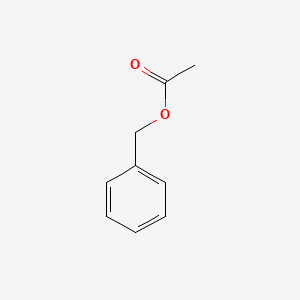 |
0.548 | D0P6UB | 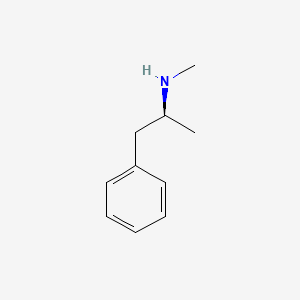 |
0.444 | ||
| ENC000597 | 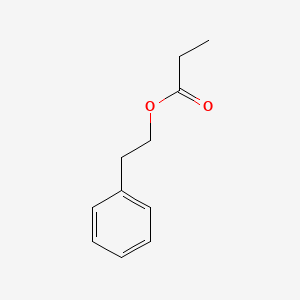 |
0.543 | D05BMG |  |
0.442 | ||
| ENC000694 | 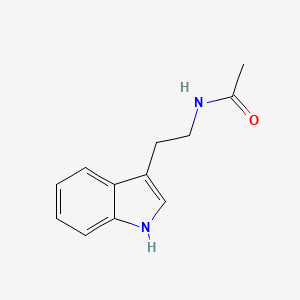 |
0.540 | D0T3LF | 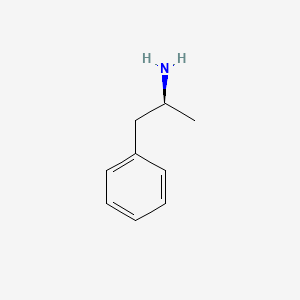 |
0.442 | ||