NPs Basic Information
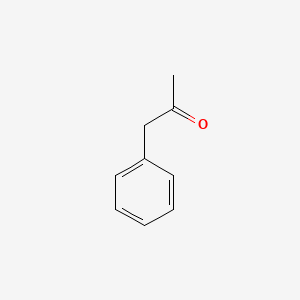
|
Name |
Phenylacetone
|
| Molecular Formula | C9H10O | |
| IUPAC Name* |
1-phenylpropan-2-one
|
|
| SMILES |
CC(=O)CC1=CC=CC=C1
|
|
| InChI |
InChI=1S/C9H10O/c1-8(10)7-9-5-3-2-4-6-9/h2-6H,7H2,1H3
|
|
| InChIKey |
QCCDLTOVEPVEJK-UHFFFAOYSA-N
|
|
| Synonyms |
Phenylacetone; 1-phenylpropan-2-one; 103-79-7; 1-Phenyl-2-propanone; Benzyl methyl ketone; Methyl benzyl ketone; Phenyl-2-propanone; 1-Phenylacetone; 2-Propanone, 1-phenyl-; 3-Phenyl-2-propanone; Phenylmethyl methyl ketone; phenyl acetone; 1-phenyl-propan-2-one; 136675-26-8; O7IZH10V9Y; CHEMBL3800510; NSC-9827; NSC 9827; Phenylacetone, 99%; EINECS 203-144-4; UNII-O7IZH10V9Y; (phenyl)acetone; AI3-02938; DEA No. 8501; methylbenzyl ketone; phenyl 2-propanone; 1-Phenylpropane-2-one; PHENYLACETONE [MI]; SCHEMBL43943; ghl.PD_Mitscher_leg0.660; DTXSID1059280; SCHEMBL13341529; CHEBI:52052; HSDB 8385; NSC9827; Phenylacetone, analytical standard; BCP22277; ZINC1700205; BDBM50167968; STL373560; AKOS004905656; FT-0673719; A800807; Q418831; AMFETAMINE SULFATE IMPURITY B [EP IMPURITY]
|
|
| CAS | 103-79-7 | |
| PubChem CID | 7678 | |
| ChEMBL ID | CHEMBL3800510 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.17 | ALogp: | 1.4 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.607 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.272 | MDCK Permeability: | 0.00004190 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.38 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.479 | Plasma Protein Binding (PPB): | 53.75% |
| Volume Distribution (VD): | 0.707 | Fu: | 32.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.531 | CYP1A2-substrate: | 0.669 |
| CYP2C19-inhibitor: | 0.685 | CYP2C19-substrate: | 0.686 |
| CYP2C9-inhibitor: | 0.161 | CYP2C9-substrate: | 0.33 |
| CYP2D6-inhibitor: | 0.01 | CYP2D6-substrate: | 0.464 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.481 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.192 | Half-life (T1/2): | 0.834 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.507 |
| Drug-inuced Liver Injury (DILI): | 0.756 | AMES Toxicity: | 0.024 |
| Rat Oral Acute Toxicity: | 0.034 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.419 | Carcinogencity: | 0.185 |
| Eye Corrosion: | 0.705 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.022 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000219 | 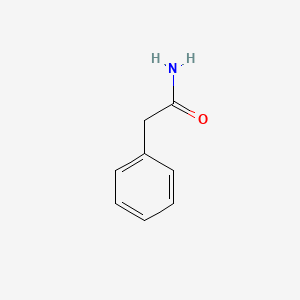 |
0.697 | D07ONP | 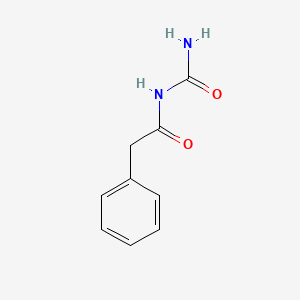 |
0.561 | ||
| ENC000054 | 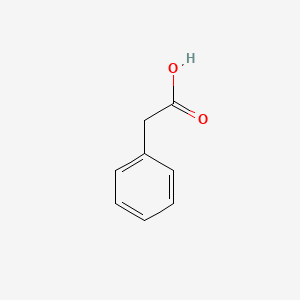 |
0.697 | D05OIS |  |
0.545 | ||
| ENC005854 |  |
0.697 | D0R1CR |  |
0.525 | ||
| ENC000208 | 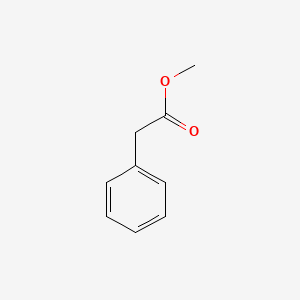 |
0.686 | D05BMG |  |
0.514 | ||
| ENC000308 | 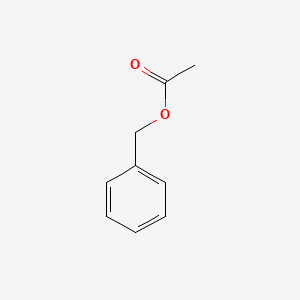 |
0.639 | D0T3LF | 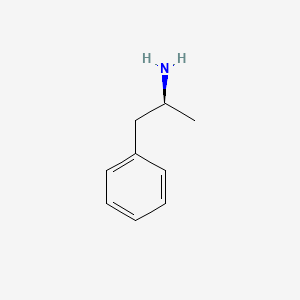 |
0.514 | ||
| ENC001819 | 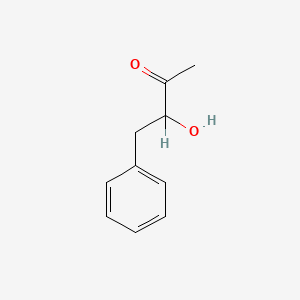 |
0.605 | D0P2GK | 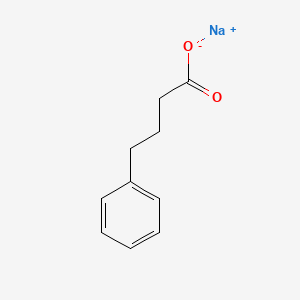 |
0.500 | ||
| ENC000203 |  |
0.594 | D0U0RZ | 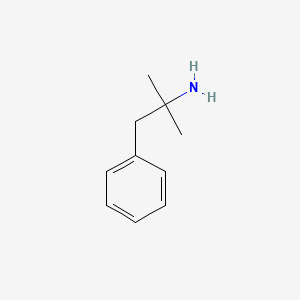 |
0.487 | ||
| ENC000216 | 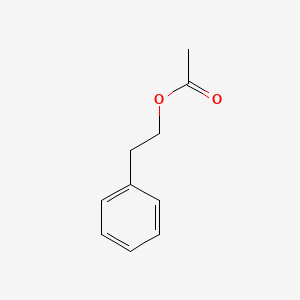 |
0.590 | D00DZN |  |
0.477 | ||
| ENC000693 | 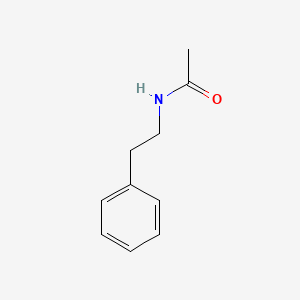 |
0.590 | D0P6UB | 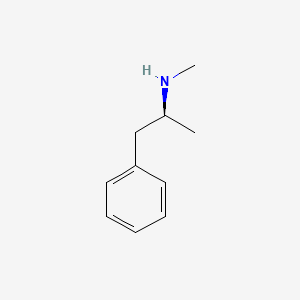 |
0.475 | ||
| ENC000192 | 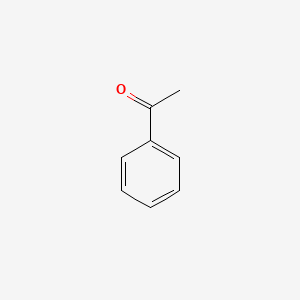 |
0.559 | D0X9RY |  |
0.472 | ||