NPs Basic Information
|
Name |
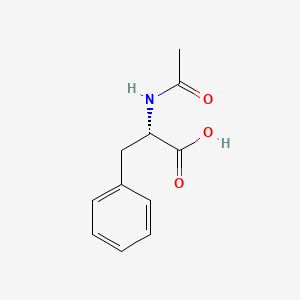
N-Acetyl-L-phenylalanine
|
| Molecular Formula | C11H13NO3 | |
| IUPAC Name* |
(2S)-2-acetamido-3-phenylpropanoic acid
|
|
| SMILES |
CC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O
|
|
| InChI |
InChI=1S/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/m0/s1
|
|
| InChIKey |
CBQJSKKFNMDLON-JTQLQIEISA-N
|
|
| Synonyms |
N-Acetyl-L-phenylalanine; 2018-61-3; Ac-Phe-OH; Acetyl-L-phenylalanine; acetylphenylalanine; N-Acetylphenylalanine; L-Phenylalanine, N-acetyl-; (S)-2-Acetamido-3-phenylpropanoic acid; L-N-Acetylphenylalanine; (2S)-2-acetamido-3-phenylpropanoic acid; Phenylalanine, N-acetyl-; N-Acetyl-3-phenyl-L-alanine; Alanine, N-acetyl-3-phenyl-, L-; N-Acetyl-l-phenalanine; NSC 45699; CHEBI:16259; NP5BT39467; MFCD00063158; UNII-NP5BT39467; NSC-45699; N-Ac-Phenylalanine; 5CR; EINECS 217-959-8; N-Ac-L-Phe; N-Acetyl Phenylalanine; N-Acetyl-Phenylalanine; n-acetyl-l-phenylalanin; N-Acetyl L-phenylalanine; Maybridge1_002391; (2S)-2-(acetylamino)-3-phenylpropanoic acid; AFALANINE, (S)-; N-Acetyl-(S)-phenylalanine; CHEMBL55743; DivK1c_001143; SCHEMBL158613; ACETYLPHENYLALANINE, L-; HMS548E15; DTXSID20883539; N-ACETYLPHENYLALANINE, L-; (+)-N-ACETYLPHENYLALANINE; HMS3264C06; Pharmakon1600-01506105; ZINC135391; ACT07471; HY-Y0068; CCG-46423; NSC760131; s6362; (+)-N-ACETYL-L-PHENYLALANINE; AKOS001051290; AKOS015837745; AM82155; CS-W020567; N-ALPHA-ACETYL-DL-PHENYLALANINE; NSC-760131; CDS1_000103; NCGC00264266-02; AC-24138; BP-12302; TS-00391; (S)-2-acetylamino-3-phenyl-propionic acid; C03519; EN300-937150; M02987; AB01209616-01; AB01209616-04; AB01209616_05; N-Acetyl-L-phenylalanine, ReagentPlus(R), 99%; 018A613; (S)-2-(ACETYLAMINO)-3-PHENYLPROPANOIC ACID; J-300205; SR-01000636121-1; W-107639; BRD-K53205568-001-01-8; Q27101819; Z56968155; N-Acetyl-L-phenylalanine, Vetec(TM) reagent grade, 98%; 545BBBE7-685F-4C55-87D5-4AB08113ECE0
|
|
| CAS | 2018-61-3 | |
| PubChem CID | 74839 | |
| ChEMBL ID | CHEMBL55743 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 207.23 | ALogp: | 0.6 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.78 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.685 | MDCK Permeability: | 0.00010864 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.062 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.498 | Plasma Protein Binding (PPB): | 24.77% |
| Volume Distribution (VD): | 0.183 | Fu: | 65.71% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.02 | CYP1A2-substrate: | 0.069 |
| CYP2C19-inhibitor: | 0.04 | CYP2C19-substrate: | 0.064 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.661 |
| CYP2D6-inhibitor: | 0.022 | CYP2D6-substrate: | 0.208 |
| CYP3A4-inhibitor: | 0.012 | CYP3A4-substrate: | 0.081 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.705 | Half-life (T1/2): | 0.768 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.226 |
| Drug-inuced Liver Injury (DILI): | 0.648 | AMES Toxicity: | 0.011 |
| Rat Oral Acute Toxicity: | 0.019 | Maximum Recommended Daily Dose: | 0.014 |
| Skin Sensitization: | 0.135 | Carcinogencity: | 0.043 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.062 |
| Respiratory Toxicity: | 0.024 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001904 | 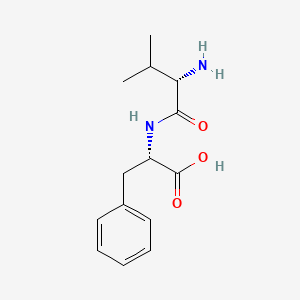 |
0.673 | D0R1CR |  |
0.574 | ||
| ENC000130 | 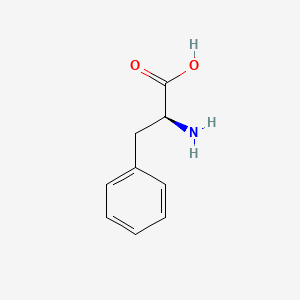 |
0.574 | D05OFX | 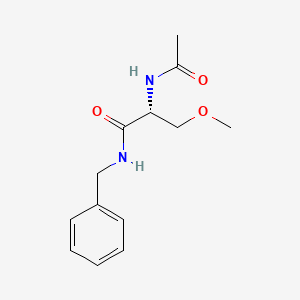 |
0.569 | ||
| ENC001819 | 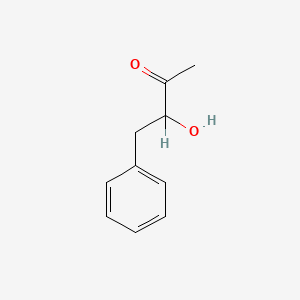 |
0.574 | D06PSS | 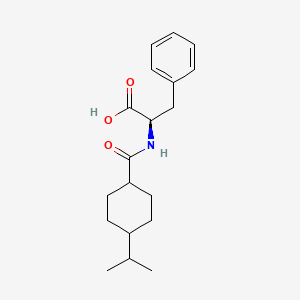 |
0.544 | ||
| ENC000218 | 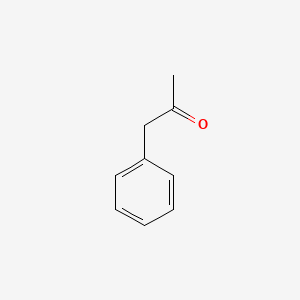 |
0.500 | D0RA5Q |  |
0.538 | ||
| ENC000054 | 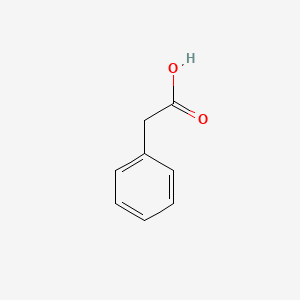 |
0.500 | D07ONP | 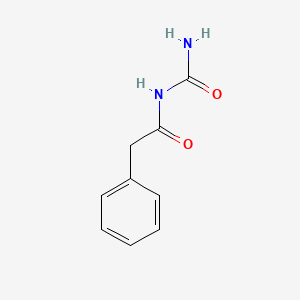 |
0.481 | ||
| ENC000693 | 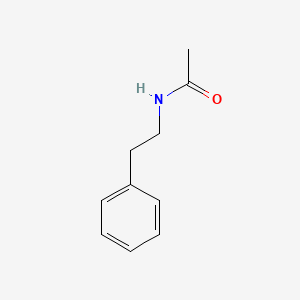 |
0.500 | D0X5SJ | 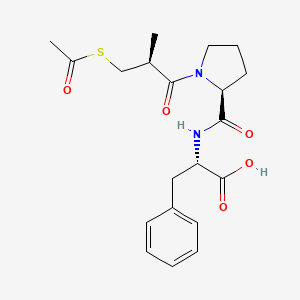 |
0.475 | ||
| ENC002126 | 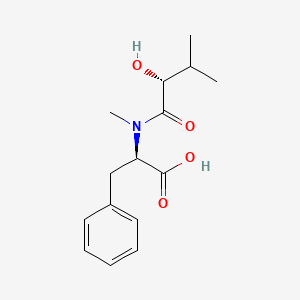 |
0.492 | D0P6UB | 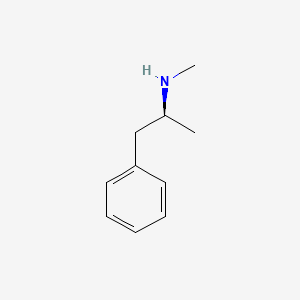 |
0.469 | ||
| ENC005220 | 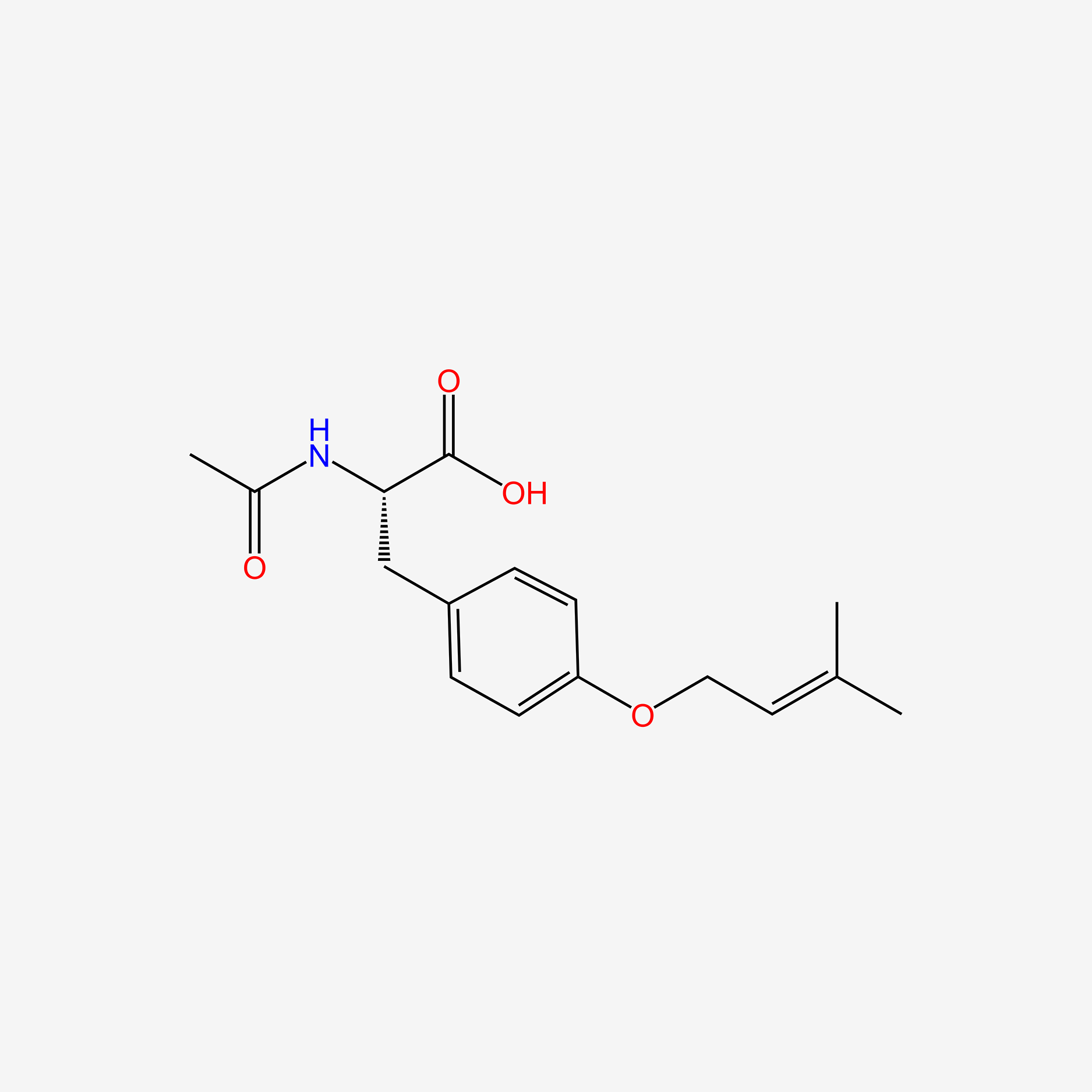 |
0.485 | D0T3LF | 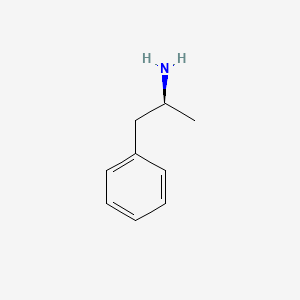 |
0.468 | ||
| ENC002014 | 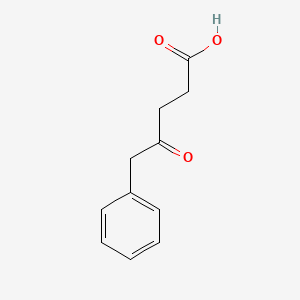 |
0.481 | D05BMG |  |
0.468 | ||
| ENC004716 | 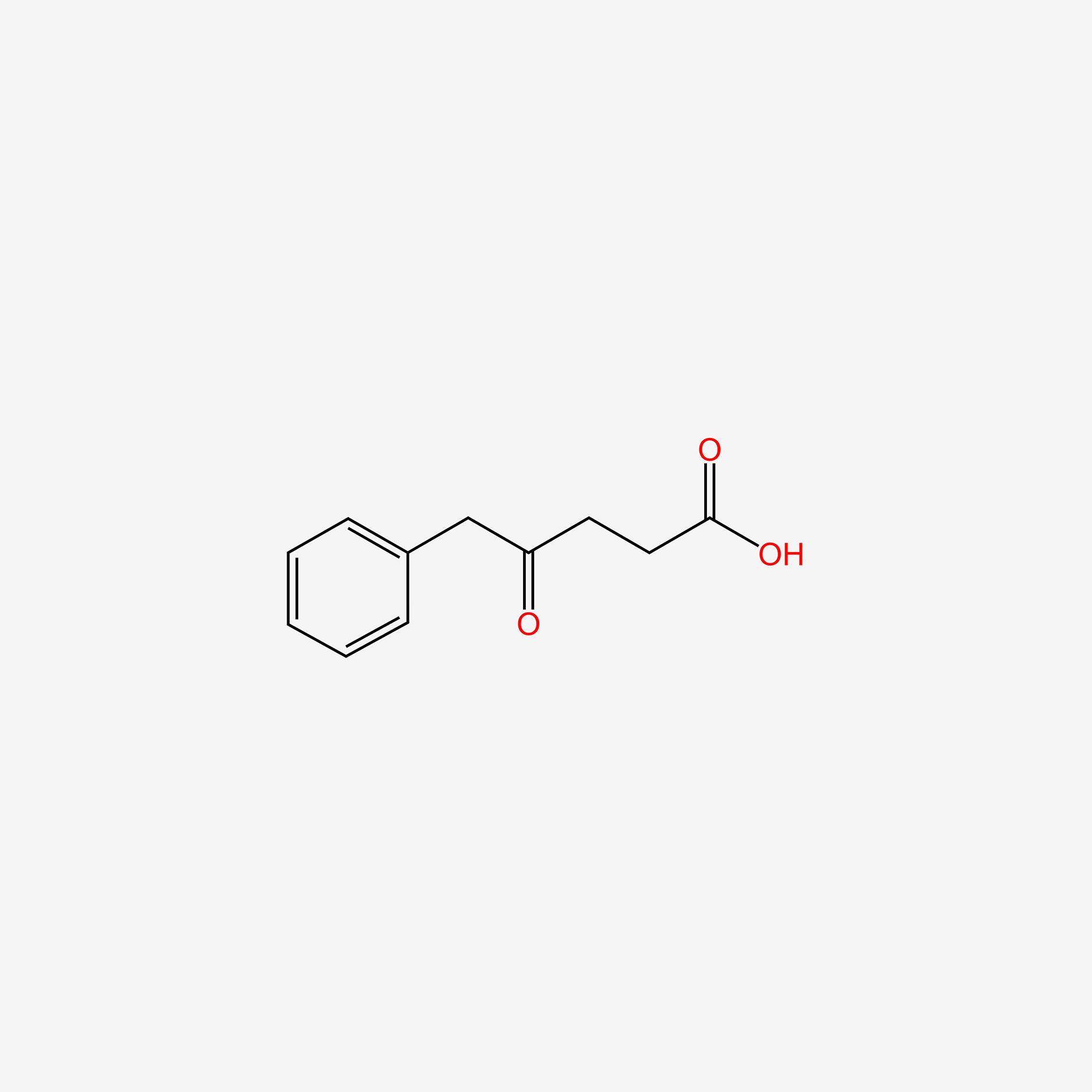 |
0.481 | D06XGW | 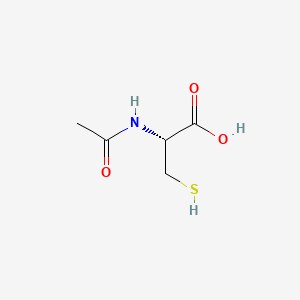 |
0.435 | ||