NPs Basic Information
|
Name |
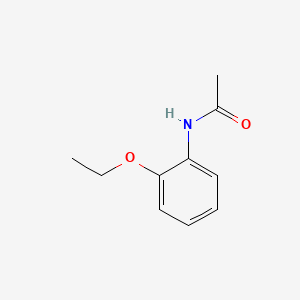
N-(2-Ethoxyphenyl)acetamide
|
| Molecular Formula | C10H13NO2 | |
| IUPAC Name* |
N-(2-ethoxyphenyl)acetamide
|
|
| SMILES |
CCOC1=CC=CC=C1NC(=O)C
|
|
| InChI |
InChI=1S/C10H13NO2/c1-3-13-10-7-5-4-6-9(10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12)
|
|
| InChIKey |
SQRTWLGWCOJOTO-UHFFFAOYSA-N
|
|
| Synonyms |
N-(2-Ethoxyphenyl)acetamide; 581-08-8; N-Acetyl-o-phenetidine; 2'-Ethoxyacetanilide; o-Acetylphenetidine; Acetamide, N-(2-ethoxyphenyl)-; 2-Ethoxyacetanilide; o-ACETOPHENETIDIDE; Acetanilide, 2'-ethoxy-; NSC 7649; o-Ethoxyacetanilide; OF0OT3R0XB; NSC-7649; EINECS 209-460-9; N-Acetyl-2-ethoxyaniline; BRN 2720676; AI3-00787; N-(2-Ethoxy-phenyl)-acetamide; NSC7649; Azetamidophenylathylather; UNII-OF0OT3R0XB; ChemDiv2_000587; o-Phenetidine, N-acetyl-; Cambridge id 5104364; WLN: 2OR BMV1; Aniline, N-acetyl-2-ethoxy-; N-(2-Ethoxyphenyl)-acetamide; SCHEMBL1645837; DTXSID7060382; ZINC30083; HMS1370K15; MFCD00059228; STK000709; AKOS000508149; AS-59116; A0103; CS-0453866; FT-0694813; D88214; A831741; AG-669/02953017
|
|
| CAS | 581-08-8 | |
| PubChem CID | 11383 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 179.22 | ALogp: | 1.5 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 38.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.774 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.21 | MDCK Permeability: | 0.00003120 |
| Pgp-inhibitor: | 0.008 | Pgp-substrate: | 0.317 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.961 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.463 | Plasma Protein Binding (PPB): | 27.17% |
| Volume Distribution (VD): | 0.962 | Fu: | 48.19% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.952 | CYP1A2-substrate: | 0.944 |
| CYP2C19-inhibitor: | 0.421 | CYP2C19-substrate: | 0.863 |
| CYP2C9-inhibitor: | 0.069 | CYP2C9-substrate: | 0.793 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.787 |
| CYP3A4-inhibitor: | 0.028 | CYP3A4-substrate: | 0.601 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.324 | Half-life (T1/2): | 0.807 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.05 | Human Hepatotoxicity (H-HT): | 0.308 |
| Drug-inuced Liver Injury (DILI): | 0.873 | AMES Toxicity: | 0.402 |
| Rat Oral Acute Toxicity: | 0.032 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.385 | Carcinogencity: | 0.561 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.718 |
| Respiratory Toxicity: | 0.026 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000106 | 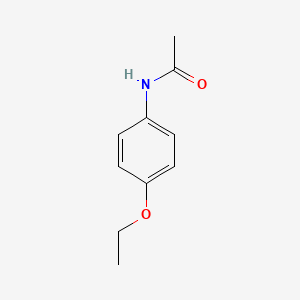 |
0.500 | D06LYG | 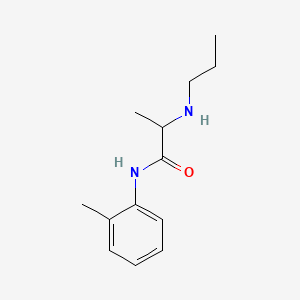 |
0.404 | ||
| ENC002891 |  |
0.449 | D0GY5Z | 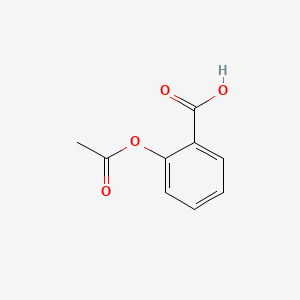 |
0.392 | ||
| ENC002235 | 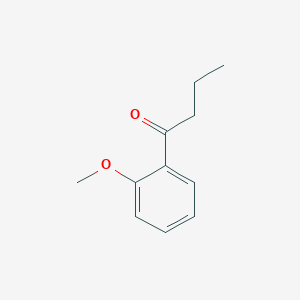 |
0.440 | D0U5QK | 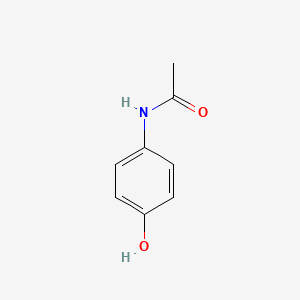 |
0.347 | ||
| ENC002213 | 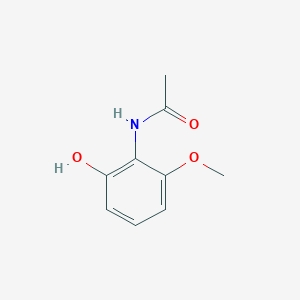 |
0.420 | D0V9JR | 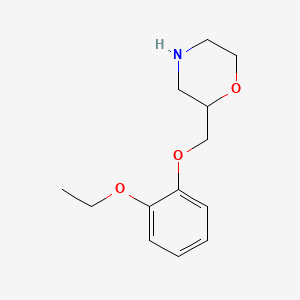 |
0.344 | ||
| ENC000823 | 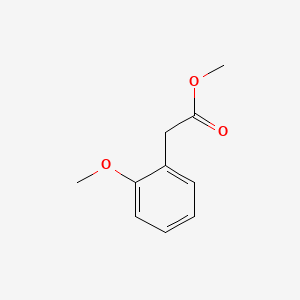 |
0.412 | D0FN7J | 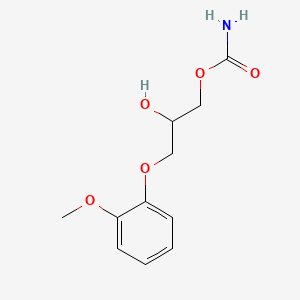 |
0.339 | ||
| ENC000160 | 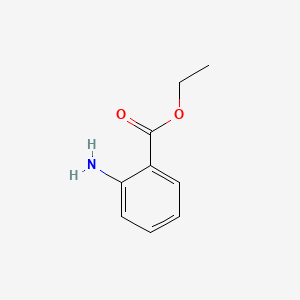 |
0.408 | D08GJO | 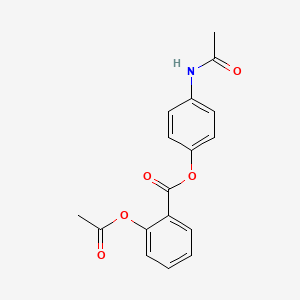 |
0.333 | ||
| ENC000370 | 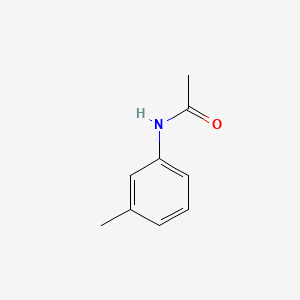 |
0.404 | D0T3NY | 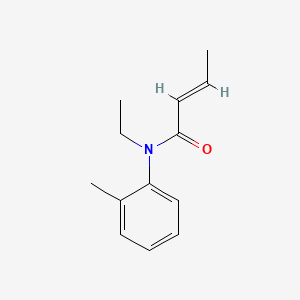 |
0.328 | ||
| ENC000175 | 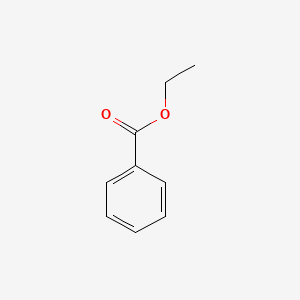 |
0.396 | D0N3UL | 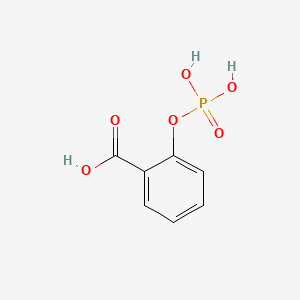 |
0.327 | ||
| ENC000073 | 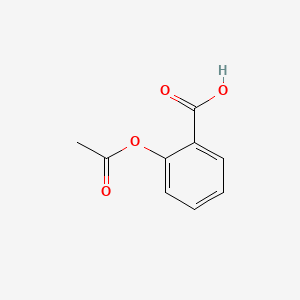 |
0.392 | D05OFX | 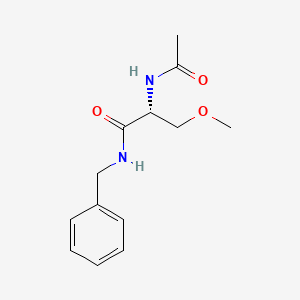 |
0.323 | ||
| ENC000693 | 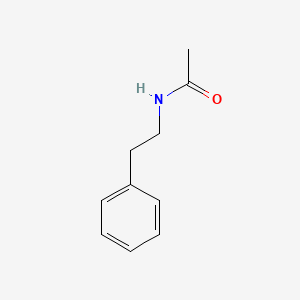 |
0.373 | D07ESC | 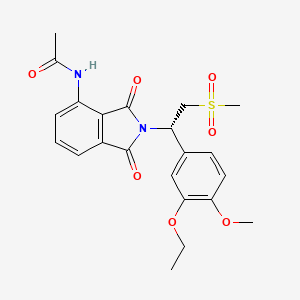 |
0.323 | ||