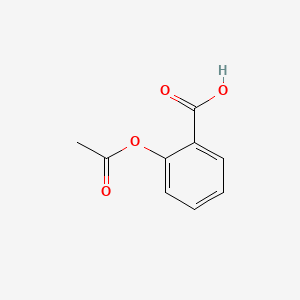
| InChI |
InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
|
| Synonyms |
aspirin; ACETYLSALICYLIC ACID; 50-78-2; 2-Acetoxybenzoic acid; 2-(Acetyloxy)benzoic acid; O-Acetylsalicylic acid; Acetylsalicylate; o-Acetoxybenzoic acid; Acylpyrin; Easprin; Ecotrin; Salicylic acid acetate; Acenterine; Acetophen; Polopiryna; Acetosal; Colfarit; o-Carboxyphenyl acetate; Acidum acetylsalicylicum; Enterosarein; Aceticyl; Acetonyl; Acetosalin; Acetylin; Aspirdrops; Benaspir; Measurin; Micristin; Pharmacin; Premaspin; Salcetogen; Temperal; Ecolen; Empirin; Endydol; Rhodine; Saletin; Rheumintabletten; Solprin acid; 2-acetyloxybenzoic acid; Benzoic acid, 2-(acetyloxy)-; Acetisal; Acetylsal; Aspirine; Bialpirina; Bialpirinia; Claradin; Clariprin; Entericin; Enterophen; Enterosarine; Globentyl; Neuronika; Salacetin; Solpyron; Acesal; Acisal; Asagran; Asteric; Cemirit; Decaten; Duramax; Extren; Globoid; Helicon; Idragin; Levius; Pirseal; Rhonal; Solfrin; Adiro; Aspec; Aspro; Novid; Yasta; Acetosalic acid; Triple-sal; Spira-Dine; ZORprin; Bi-prin; Acetilum acidulatum; Acimetten; Delgesic; Entrophen; Persistin; 2-Carboxyphenyl acetate; Acetilsalicilico; Dolean pH 8; A.S.A. empirin; XAXA; Acido acetilsalicilico; Contrheuma retard; Acide acetylsalicylique; ASA; Endosprin; Kapsazal; Bayer; Acetylsalicylsaure; aspirin (acetylsalicylic acid); Solprin; Triaminicin; Asatard; Durlaza; Tasprin; Nu-seals aspirin; Salicylic acid, acetate; Acido O-acetil-benzoico; Kyselina acetylsalicylova; 2-Acetoxybenzenecarboxylic acid; St. Joseph Aspirin for Adults; A.S.A.; St. Joseph; Kyselina 2-acetoxybenzoova; SP 189; Acetard; AC 5230; acetyl salicylate; Acetylsalicylsaeure; Azetylsalizylsaeure; S-211; ECM; CHEBI:15365; 2-(acetyloxy)benzoate; Acetylsalicylicum acidum; NSC-27223; o-(Acetyloxy)benzoic acid; NSC-406186; acide 2-(acetyloxy)benzoique; Acetylsalicylic acid (who-ip); R16CO5Y76E; Aspirin form II; component of Midol; NSC27223; component of Synirin; 8-hour Bayer; component of Zactirin; BAY1019036; component of Coricidin; component of Persistin; component of Robaxisal; o-Acetoxybenzoate; NCGC00015067-04; Acetysal; Istopirin; Magnecyl; Medisyl; Polopirin; Ronal; Bayer Buffered; DSSTox_CID_108; Aspro Clear; component of Ascodeen-30; Bayer Plus; WLN: QVR BOV1; Rheumin tabletten; AcetylsalicylicAcid; DSSTox_RID_75372; DSSTox_GSID_20108; Aspirina 03; Acetylsalycilic acid; acetyl salicylic acid; component of Darvon with A.S.A; Bayer Aspirin 8 Hour; Asaphen; Aspalon; Asprin; Bayer Children's Aspirin; Nu-seals; component of St. Joseph Cold Tablets; Aspir-Mox; Durlaza ER; Acetylsalicylsaure [German]; CAS-50-78-2; Acetoxybenzoic acid; Acetysalicylic acid; 11126-35-5; AIN; SMR000059138; Ascoden-30; Benzoicacid, 2-(acetyloxy)-; Acetylsalicyclic acid; CCRIS 3243; HSDB 652; Acide acetylsalicylique [French]; Acido acetilsalicilico [Italian]; Kyselina acetylsalicylova [Czech]; Acido O-acetil-benzoico [Italian]; SR-01000075668; Kyselina 2-acetoxybenzoova [Czech]; Bayer Extra Strength Aspirin for Migraine Pain; EINECS 200-064-1; NSC 27223; Aspirin [USP:BAN:JAN]; Bayer Enteric 325 mg Regular Strength; BRN 0779271; Bay E4465; UNII-R16CO5Y76E; Aspropharm; Bayer Enteric 81 mg Adult Low Strength; Cardioaspirin; Cardioaspirina; Acetyonyl; Asacard; Ascolong; Bayer Enteric 500 mg Arthritis Strength; Colsprin; Miniasal; Salospir; Acesan; Toldex; AI3-02956; 1oxr; 2-Acetoxybenzoate; Aspirin,(S); Aspalon (JAN); Durlaza (TN); Easprin (TN); MFCD00002430; acetyl-salicylic acid; Aspirin USP-26; acetyl salicyclic acid; o-(Acetyloxy)benzoate; Percodan (Salt/Mix); Ascriptin (Salt/Mix); Micrainin (Salt/Mix); 2-acetoxy benzoic acid; Spectrum_001245; 2-Acetylsalicyclic acid; ASPIRIN [VANDF]; ASPIRIN [HSDB]; Salicylic acid, acetyl-; ASPIRIN [JAN]; ASPIRIN [MI]; CHEMBL25; ASPIRIN [MART.]; Spectrum2_001899; Spectrum3_001295; Spectrum4_000099; Spectrum5_000740; Aspirin (JP17/USP); Lopac-A-5376; Salycylacetylsalicylic acid; ASPIRIN [USP-RS]; benzoic acid, 2-acetoxy-; Epitope ID:114151; Percodan Demi (Salt/Mix); Soma Compound (Salt/Mix); ZINC53; EC 200-064-1; Acetylsalicylic acid, 99%; Aspirin USP (3080); cid_2244; Pravigard PAC (Salt/Mix); SCHEMBL1353; 2-(Acetyloxy)-benzoic acid; Aspirin USP (2080B); Bay-e-4465; Acetylsalicylic acid-[13C]; Lopac0_000038; KBioGR_000398; KBioGR_002271; KBioSS_001725; KBioSS_002272; 4-10-00-00138 (Beilstein Handbook Reference); MLS001055329; MLS001066332; MLS001336045; MLS001336046; ASPIRIN [ORANGE BOOK]; BIDD:GT0118; DivK1c_000555; SPECTRUM1500130; SPBio_001838; Acetylsalicylic acid, >=99%; AXOTAL COMPONENT ASPIRIN; AZDONE COMPONENT ASPIRIN; CODOXY COMPONENT ASPIRIN; GTPL4139; (non-d)Acetylsalicylic Acid-d3; ASPIRIN [USP MONOGRAPH]; O-Acetylsalicylic acid; Aspirin; DTXSID5020108; Acetylsalicylic acid-carboxy-14c; AGGRENOX COMPONENT ASPIRIN; BDBM22360; DUOCOVER COMPONENT ASPIRIN; EXCEDRIN COMPONENT ASPIRIN; FIORINAL COMPONENT ASPIRIN; HMS501L17; KBio1_000555; KBio2_001725; KBio2_002271; KBio2_004293; KBio2_004839; KBio2_006861; KBio2_007407; KBio3_002149; KBio3_002751; NORGESIC COMPONENT ASPIRIN; PERCODAN COMPONENT ASPIRIN; Q-GESIC COMPONENT ASPIRIN; ROXIPRIN COMPONENT ASPIRIN; VICOPRIN COMPONENT ASPIRIN; YOSPRALA COMPONENT ASPIRIN; Empirin with Codeine (Salt/Mix); Acetylsalicylic acid, >=99.0%; cMAP_000006; component of Zactirin (Salt/Mix); DUOPLAVIN COMPONENT ASPIRIN; EQUAGESIC COMPONENT ASPIRIN; INVAGESIC COMPONENT ASPIRIN; LANORINAL COMPONENT ASPIRIN; MICRAININ COMPONENT ASPIRIN; NINDS_000555; ROBAXISAL COMPONENT ASPIRIN; ASPIRIN COMPONENT OF AXOTAL; ASPIRIN COMPONENT OF AZDONE; ASPIRIN COMPONENT OF CODOXY; HMS1920E13; HMS2090G03; HMS2091K13; HMS2233L18; HMS3260G17; HMS3372N15; HMS3656N14; HMS3715P19; HMS3866L03; HMS3885G03; Pharmakon1600-01500130; ACETYLSALICYLIC ACID [INCI]; BCP21790; ORPHENGESIC COMPONENT ASPIRIN; STR01551; ACETYLSALICYLIC ACID; ASPIRIN; ASPIRIN COMPONENT OF AGGRENOX; ASPIRIN COMPONENT OF DUOCOVER; ASPIRIN COMPONENT OF EXCEDRIN; ASPIRIN COMPONENT OF FIORINAL; ASPIRIN COMPONENT OF NORGESIC; ASPIRIN COMPONENT OF PERCODAN; ASPIRIN COMPONENT OF Q-GESIC; ASPIRIN COMPONENT OF ROXIPRIN; ASPIRIN COMPONENT OF VICOPRIN; ASPIRIN COMPONENT OF YOSPRALA; SYNALGOS-DC COMPONENT ASPIRIN; Tox21_110076; Tox21_202117; Tox21_300146; Tox21_500038; CCG-39490; NSC406186; NSC755899; s3017; STL137674; ACETYLSALICYLIC ACID [WHO-DD]; ASPIRIN COMPONENT OF DUOPLAVIN; ASPIRIN COMPONENT OF EQUAGESIC; ASPIRIN COMPONENT OF INVAGESIC; ASPIRIN COMPONENT OF LANORINAL; ASPIRIN COMPONENT OF MICRAININ; ASPIRIN COMPONENT OF ROBAXISAL; AKOS000118884; component of Ascodeen-30 (Salt/Mix); MEPRO-ASPIRIN COMPONENT ASPIRIN; PERCODAN-DEMI COMPONENT ASPIRIN; PRAVIGARD PAC COMPONENT ASPIRIN; SOMA COMPOUND COMPONENT ASPIRIN; Tox21_110076_1; ACETYLSALICYLIC ACID [EMA EPAR]; ACETYLSALICYLICUM ACIDUM [HPUS]; ASPIRIN COMPONENT OF ORPHENGESIC; CS-2001; DB00945; LP00038; NSC-755899; PL-2200; SDCCGSBI-0050027.P005; ASPIRIN COMPONENT OF SYNALGOS-DC; BAY-1019036; DARVON COMPOUND COMPONENT ASPIRIN; IDI1_000555; INVAGESIC FORTE COMPONENT ASPIRIN; TALWIN COMPOUND COMPONENT ASPIRIN; ACETYLSALICYLIC ACID [GREEN BOOK]; Acetylsalicylic acid, analytical standard; ACIDUM ACETYLSALICYLICUM (WHO-IP); NCGC00015067-01; NCGC00015067-02; NCGC00015067-03; NCGC00015067-05; NCGC00015067-06; NCGC00015067-07; NCGC00015067-08; NCGC00015067-09; NCGC00015067-10; NCGC00015067-11; NCGC00015067-12; NCGC00015067-13; NCGC00015067-14; NCGC00015067-24; NCGC00015067-26; NCGC00090977-01; NCGC00090977-02; NCGC00090977-03; NCGC00090977-04; NCGC00090977-05; NCGC00090977-06; NCGC00090977-07; NCGC00254034-01; NCGC00259666-01; NCGC00260723-01; ASPIRIN COMPONENT OF MEPRO-ASPIRIN; ASPIRIN COMPONENT OF PERCODAN-DEMI; ASPIRIN COMPONENT OF PRAVIGARD PAC; ASPIRIN COMPONENT OF SOMA COMPOUND; Aspirin, meets USP testing specifications; HY-14654; NCI60_002222; ORPHENGESIC FORTE COMPONENT ASPIRIN; ACETYLSALICYLIC ACID [EP MONOGRAPH]; SBI-0050027.P004; ASPIRIN COMPONENT OF DARVON COMPOUND; ASPIRIN COMPONENT OF INVAGESIC FORTE; ASPIRIN COMPONENT OF TALWIN COMPOUND; DS-017139; UNM-0000306102; ASPIRIN COMPONENT OF ORPHENGESIC FORTE; component of Darvon with A.S.A (Salt/Mix); EU-0100038; FT-0655181; FT-0661360; SW199665-2; CARISOPRODOL COMPOUND COMPONENT ASPIRIN; EN300-19606; A 5376; Acetylsalicylic Acid 1.0 mg/ml in Acetonitrile; C01405; D00109; E80792; Q18216; AB00051918-08; AB00051918_09; AB00051918_10; ASPIRIN COMPONENT OF CARISOPRODOL COMPOUND; Arthritis Pain Formula Maximum Strength (Salt/Mix); SR-01000075668-1; SR-01000075668-4; SR-01000075668-6; Acetylsalicylic acid, Vetec(TM) reagent grade, >=99%; Aspirin, British Pharmacopoeia (BP) Reference Standard; CLOPIDOGREL/ACETYLSALICYLIC ACID COMPONENT ASPIRIN; F2191-0068; Z104474430; ASPIRIN COMPONENT OF CLOPIDOGREL/ACETYLSALICYLIC ACID; Aspirin, United States Pharmacopeia (USP) Reference Standard; D41527A7-A9EB-472D-A7FC-312821130549; Acetylsalicylic acid, European Pharmacopoeia (EP) Reference Standard; Acetylsalicylic acid, BioReagent, plant cell culture tested, >=99.0%; Acetylsalicylic acid for peak identification, European Pharmacopoeia (EP) Reference Standard; Aspirin (Acetyl Salicylic Acid), Pharmaceutical Secondary Standard; Certified Reference Material
|