NPs Basic Information

|
Name |
Indole-3-carboxaldehyde
|
| Molecular Formula | C9H7NO | |
| IUPAC Name* |
1H-indole-3-carbaldehyde
|
|
| SMILES |
C1=CC=C2C(=C1)C(=CN2)C=O
|
|
| InChI |
InChI=1S/C9H7NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-6,10H
|
|
| InChIKey |
OLNJUISKUQQNIM-UHFFFAOYSA-N
|
|
| Synonyms |
INDOLE-3-CARBOXALDEHYDE; 1H-Indole-3-carbaldehyde; 487-89-8; 3-Formylindole; Indole-3-carbaldehyde; 1H-Indole-3-carboxaldehyde; Indole-3-aldehyde; 3-Indolecarboxaldehyde; INDOLE-3-CARBOXYALDEHYDE; Indol-3-carboxaldehyde; beta-Indolylaldehyde; 3-Indolecarbaldehyde; 3-Indolealdehyde; Indol-3-carbaldehyde; MFCD00005622; NSC 10118; 3-indolylformaldehyde; 4877-89-8; .beta.-Indolylaldehyde; 1H-indole-3-aldehyde; 7FN04C32UO; CHEMBL147741; NSC-10118; 3-Formylindol; 3-indolemethanal; Indol-3-carbaldehyd; 3-Formyl-1H-indole; EINECS 207-665-8; BRN 0114117; UNII-7FN04C32UO; AI3-52407; b-Indolylaldehyde; 3-formyl indole; 3-formyl-indole; A-Indolylaldehyde; Indol-3-aldehyde; 3-indole aldehyde; indolyl-3-aldehyde; indole-3-carboaldehyde; indole 3-carboxaldehyde; I3CHO; I3CA; 1H-indole-3-carbaldehyd; h-indole-3-carboxaldehyde; indole-3-carboxy-aldehyde; 1H-Indole-3-carboxaldehde; bmse000645; SCHEMBL56373; 5-21-08-00246 (Beilstein Handbook Reference); Indole-3-carboxaldehyde, 97%; DTXSID5060069; CHEBI:28238; OLNJUISKUQQNIM-UHFFFAOYSA-; ZINC87959; ACT03589; BCP00081; NSC10118; AM1029; BDBM50182880; STK387546; AKOS000119898; CS-W007376; HY-W007376; PS-5323; SB14957; NCGC00161738-01; NCGC00161738-02; AC-23425; DB-011568; A7354; BB 0242392; FT-0615872; FT-0652575; FT-0670335; I0027; EN300-16816; 87I898; C08493; I-2200; AB00443651-03; Indole-3-carboxaldehyde, purum, >=98.0% (T); A827605; A871878; A897853; AG-205/01412034; CU-00000000108-1; Q27103575; Z56785575; F0918-0115; 3-Indolylformaldehyde, 3-Formylindole, Indole-3-carbaldehyde; 1228547-52-1; 1H-indole-3-carbaldehyde1H-Indole-3-carboxaldehyde487-89-8246045-99-8.beta.-IndolylaldehydeIndole-3-carbaldehydeIndole-3-carboxaldehyde57210_FLUKA129445_ALDRICHZINC00087959SBB004120BAS 07339836C084933
|
|
| CAS | 487-89-8 | |
| PubChem CID | 10256 | |
| ChEMBL ID | CHEMBL147741 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 145.16 | ALogp: | 1.7 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 32.9 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.615 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.492 | MDCK Permeability: | 0.00000989 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.967 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.899 | Plasma Protein Binding (PPB): | 81.64% |
| Volume Distribution (VD): | 1.172 | Fu: | 19.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.977 | CYP1A2-substrate: | 0.45 |
| CYP2C19-inhibitor: | 0.559 | CYP2C19-substrate: | 0.285 |
| CYP2C9-inhibitor: | 0.12 | CYP2C9-substrate: | 0.911 |
| CYP2D6-inhibitor: | 0.081 | CYP2D6-substrate: | 0.782 |
| CYP3A4-inhibitor: | 0.105 | CYP3A4-substrate: | 0.182 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.548 | Half-life (T1/2): | 0.798 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.128 |
| Drug-inuced Liver Injury (DILI): | 0.2 | AMES Toxicity: | 0.095 |
| Rat Oral Acute Toxicity: | 0.465 | Maximum Recommended Daily Dose: | 0.517 |
| Skin Sensitization: | 0.878 | Carcinogencity: | 0.25 |
| Eye Corrosion: | 0.912 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.977 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000042 | 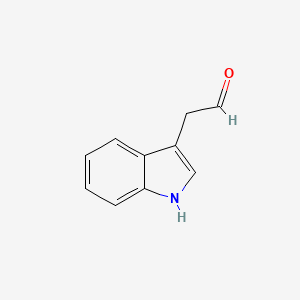 |
0.634 | D05EJG | 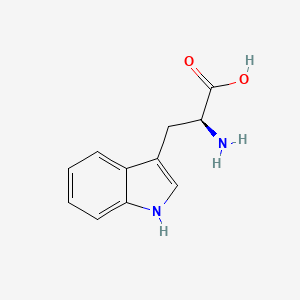 |
0.480 | ||
| ENC005757 | 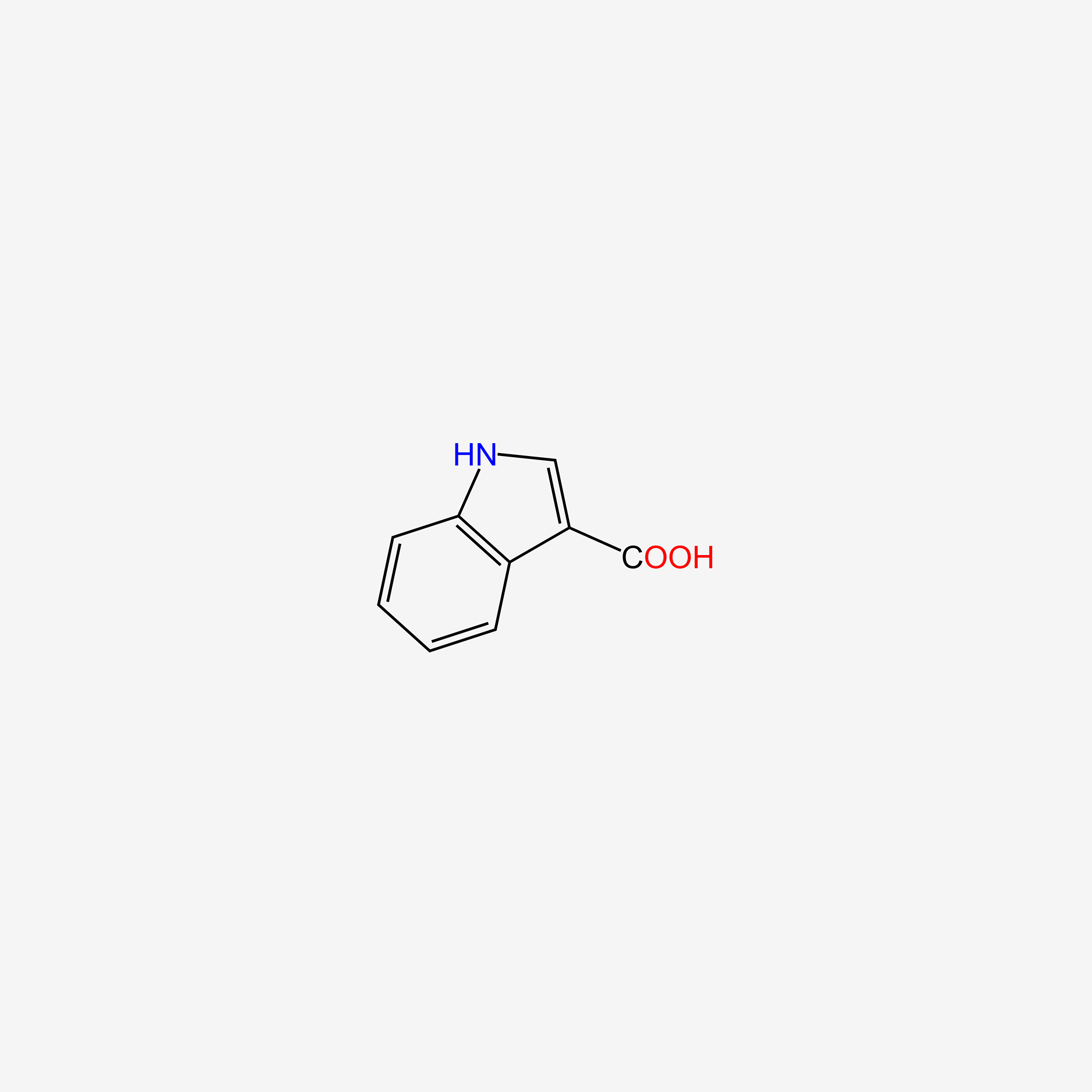 |
0.571 | D0K0KH | 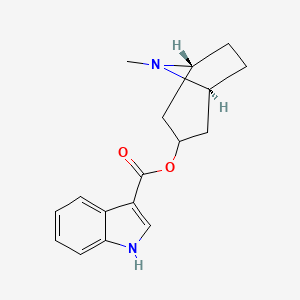 |
0.348 | ||
| ENC001345 |  |
0.533 | D0K1XK |  |
0.314 | ||
| ENC000999 |  |
0.533 | D00YLW |  |
0.308 | ||
| ENC000043 | 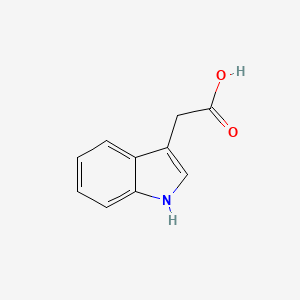 |
0.533 | D08QCJ | 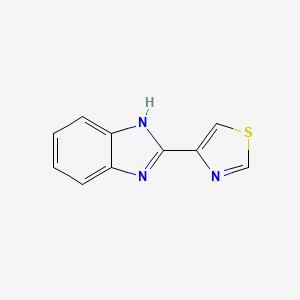 |
0.298 | ||
| ENC000363 | 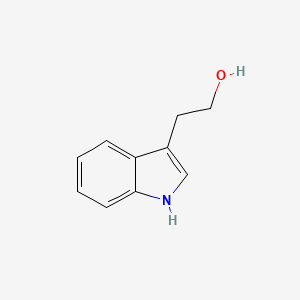 |
0.523 | D0X9RY |  |
0.295 | ||
| ENC001448 | 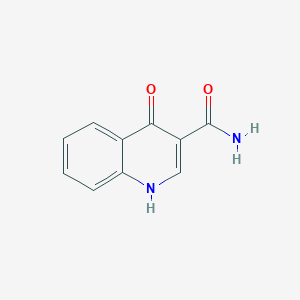 |
0.511 | D09ZIS | 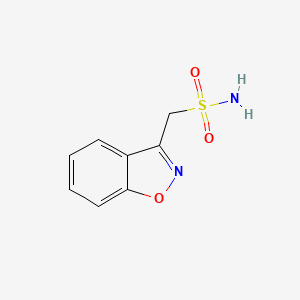 |
0.291 | ||
| ENC000140 | 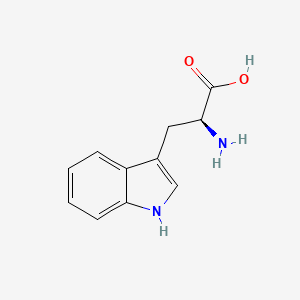 |
0.480 | D01ZJK |  |
0.286 | ||
| ENC004706 | 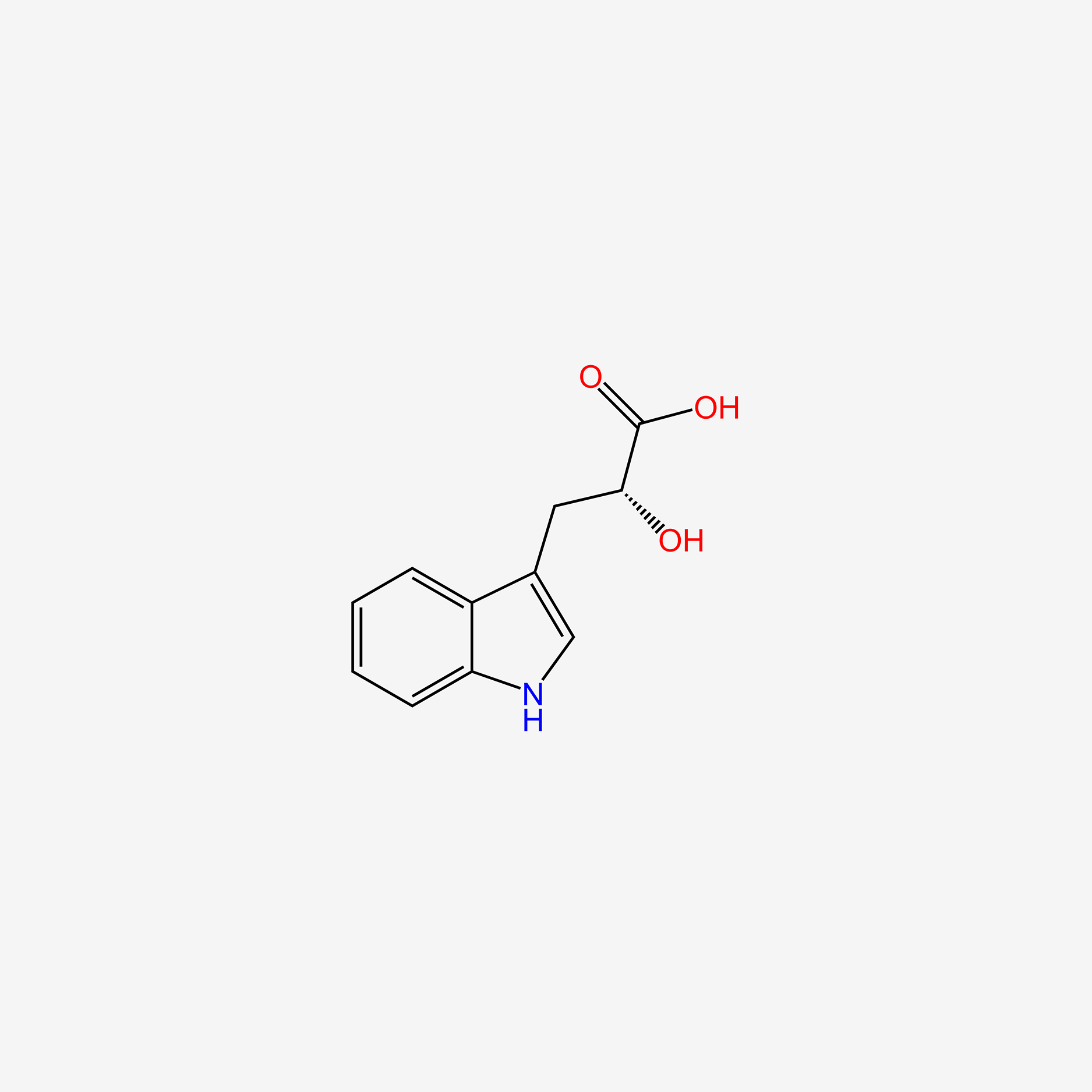 |
0.480 | D03GET | 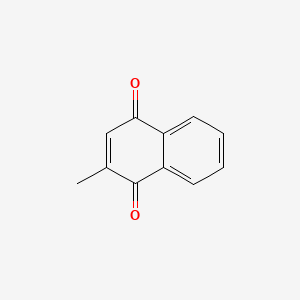 |
0.283 | ||
| ENC000694 |  |
0.471 | D07HBX |  |
0.283 | ||