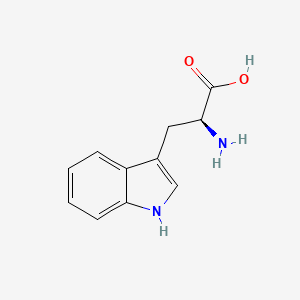
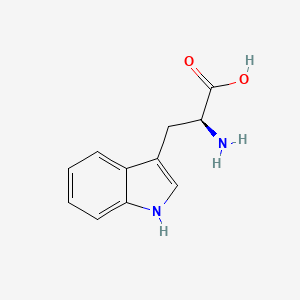
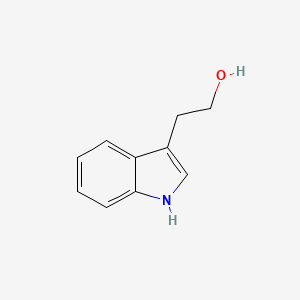
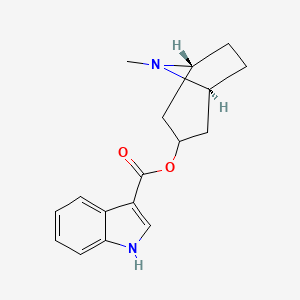
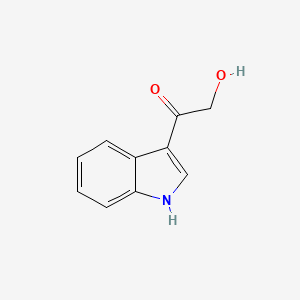
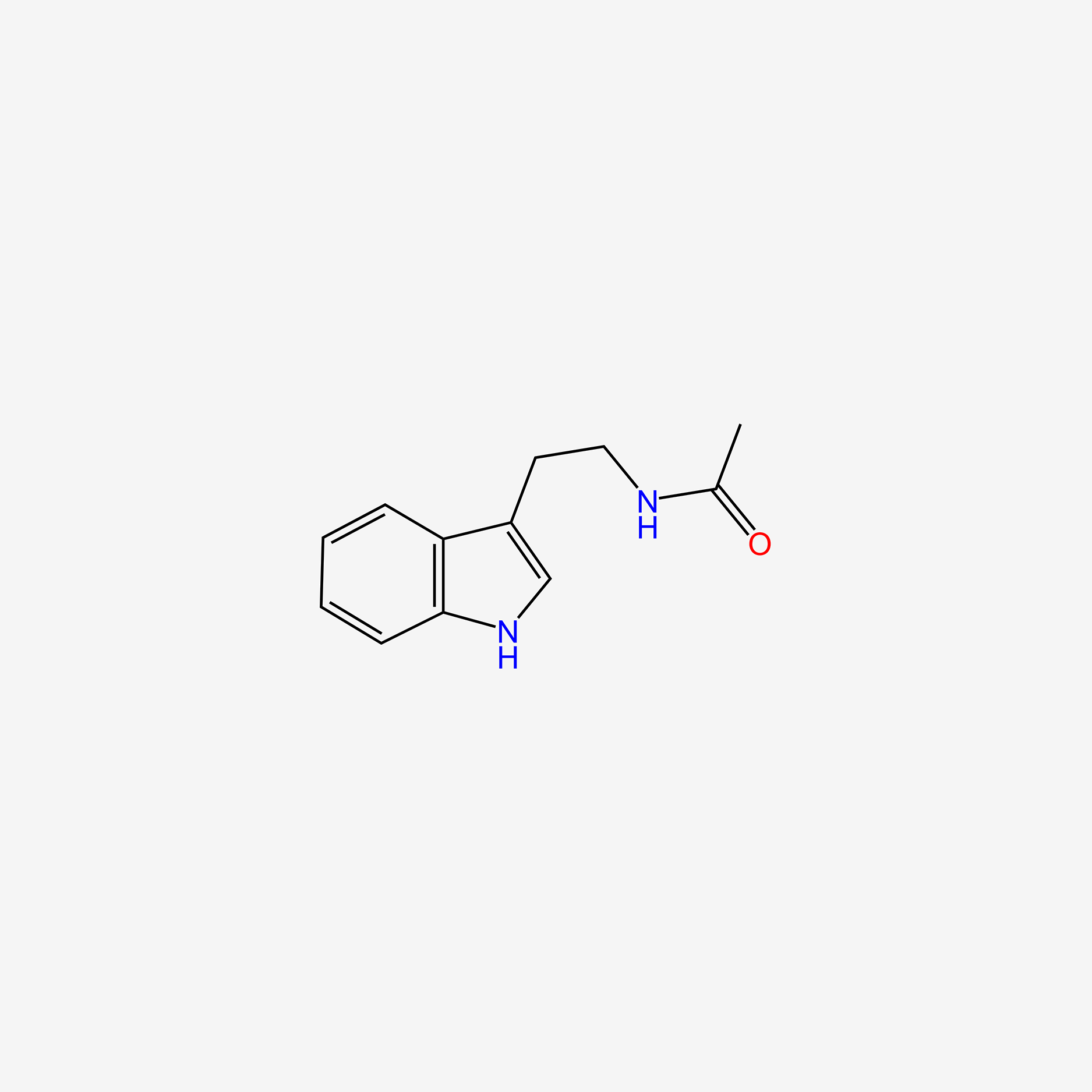
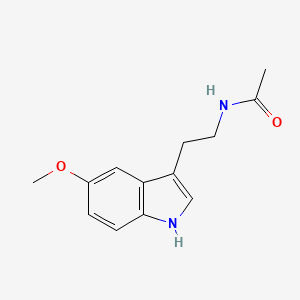
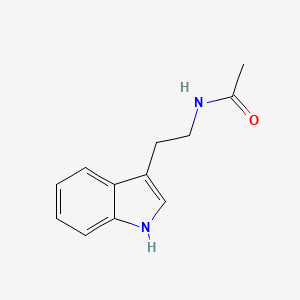
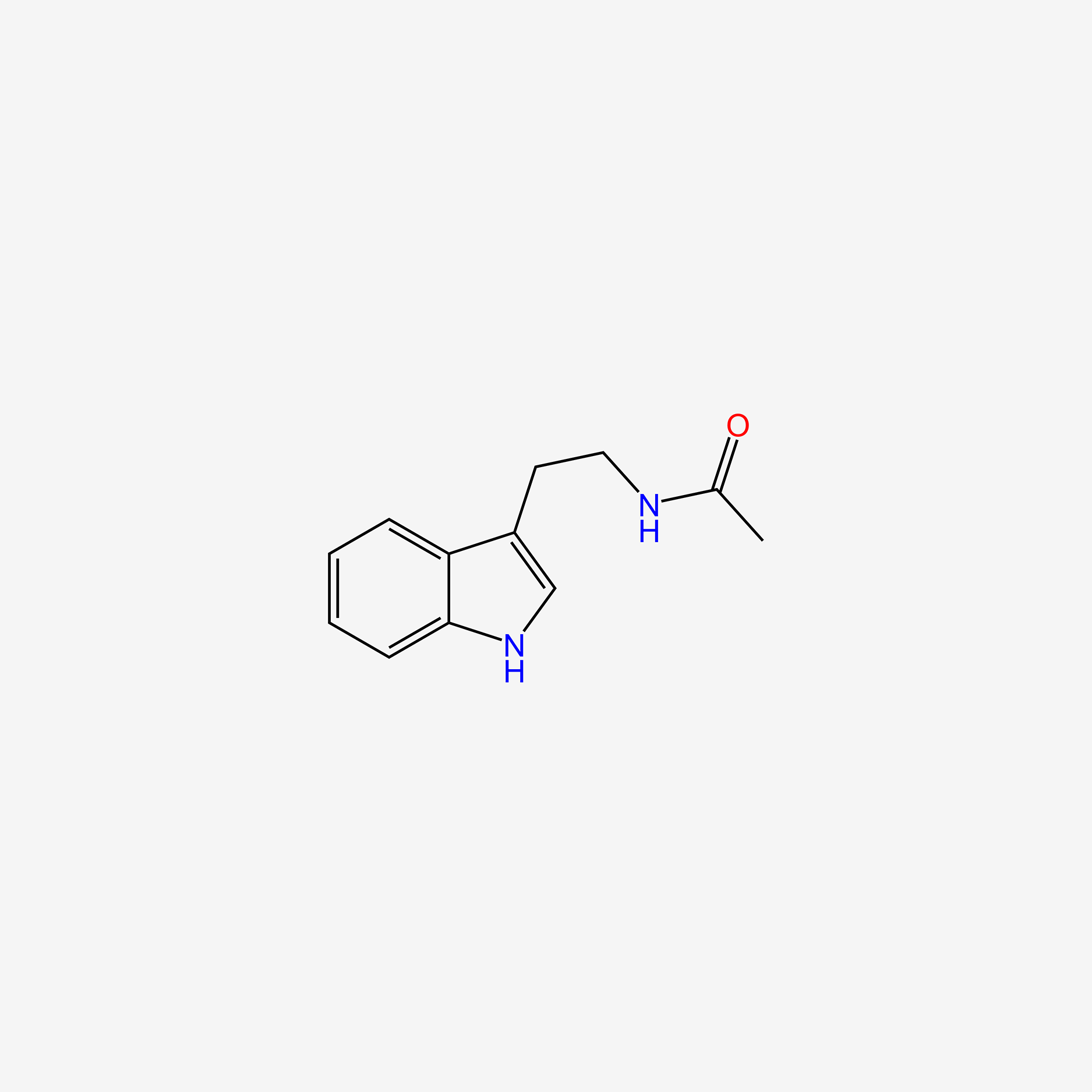
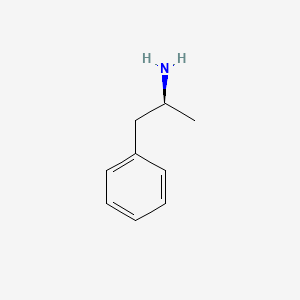
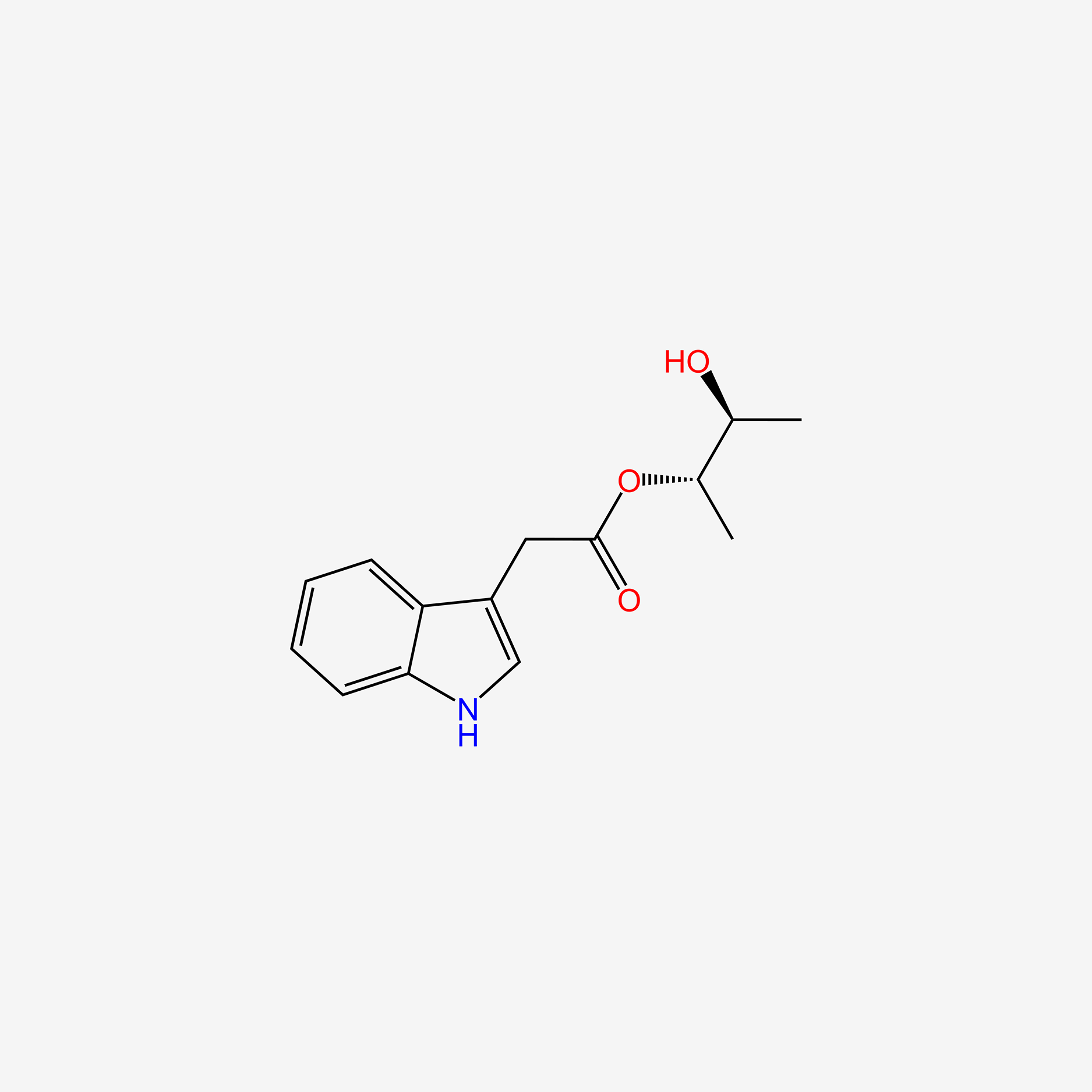
| InChI |
InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
| Synonyms |
L-tryptophan; tryptophan; 73-22-3; L-Tryptophane; h-Trp-oh; (S)-Tryptophan; Tryptophane; trofan; tryptacin; Optimax; Ardeytropin; (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid; Pacitron; Indole-3-alanine; Kalma; L-beta-3-Indolylalanine; L-Tryptofan; L-Trp; L-(-)-Tryptophan; 3-Indol-3-ylalanine; Tryptan; Lyphan; Tryptophan (VAN); 1-beta-3-Indolylalanine; Tryptophan (H-3); Triptofano [Spanish]; Tryptophanum [Latin]; 1H-Indole-3-alanine; Tryptophan, L-; 1beta-3-Indolylalanine; (-)-Tryptophan; 2-Amino-3-indolylpropanoic acid; L(-)-Tryptophan; triptofano; Tryptophanum; (S)-alpha-Amino-1H-indole-3-propanoic acid; Tryptophane [French]; (L)-TRYPTOPHAN; alpha'-Amino-3-indolepropionic acid; Tryptophan [USAN:INN]; L-alpha-amino-3-indolepropionic acid; L-alpha-Aminoindole-3-propionic acid; Sedanoct; (S)-alpha-Aminoindole-3-propionic acid; 1H-Indole-3-alanine (VAN); EH 121; trp; Alanine, 3-indol-3-yl-; CCRIS 617; L-Alanine, 3-(1H-indol-3-yl)-; 1H-Indole-3-alanine, (S)-; alpha-Amino-3-indolepropionic acid, L-; HSDB 4142; Trytophan-; (S)-alpha-amino-beta-(3-indolyl)-propionic acid; NCI-C01729; AI3-18478; (S)-2-Amino-3-(3-indolyl)propionic acid; Indole-3-propionic acid, alpha-amino-; 1H-Indole-3-propanoic acid, alpha-amino-, (S)-; Propionic acid, 2-amino-3-indol-3-yl-; CHEBI:16828; Lopac-T-0254; Tryptophan ((-),l,s); 8DUH1N11BX; (S)-alpha-Amino-beta-indolepropionic acid; CHEMBL54976; (S)-2-Amino-3-(1H-indol-3-yl)propanoic acid; NSC-13119; MFCD00064340; DSSTox_CID_1419; DSSTox_RID_76152; DSSTox_GSID_21419; 80206-30-0; l-b-3-Indolylalanine; L-Tryptophan-13C11,15N2; D-Trp-OH; CAS-73-22-3; Propionic acid, 2-amino-3-indol-3-yl; L-Tryptophan (9CI); Tryptophan (USP/INN); (S)-a-Amino-b-indolepropionic acid; (S)-a-Aminoindole-3-propionic acid; Alanine, 3-indol-3-yl; EINECS 200-795-6; NSC 13119; UNII-8DUH1N11BX; (2S)-2-amino-3-(1H-indol-3-yl)propanoate; trytophan; (S)-a-Amino-1H-indole-3-propanoic acid; TRP-01; L-Trytophan; 1qaw; L-Tryptophan,(S); L-Trp-OH; 2a4m; H-L-Trp-OH; TRYPTOPHAN [II]; TRYPTOPHAN [MI]; L-Tryptophan (JP17); TRYPTOPHAN [INN]; S(-)-1-alpha-Aminoindole-3-propionic acid; TRYPTOPHAN [HSDB]; TRYPTOPHAN [INCI]; TRYPTOPHAN [USAN]; Tryptophan (L-Tryptophan); TRYPTOPHAN [VANDF]; Tryptophan, L- (8CI); bmse000050; bmse000868; bmse001017; Epitope ID:136043; EC 200-795-6; T 0254; L-TRYPTOPHAN [FCC]; L-TRYPTOPHAN [JAN]; SCHEMBL7328; TRYPTOPHAN [MART.]; 2-Amino-3-indolylpropanoate; (S)-(-)-2-Amino-3-(3-indolyl)propionic Acid; (S)-1H-Indole-3-alanine; Lopac0_001183; GTPL717; L-TRYPTOPHAN [VANDF]; MLS001056750; DivK1c_000457; L-TRYPTOPHAN [USP-RS]; (s)-a-amino-b-indolepropionate; 151A3008-4CFE-40C9-AC0B-467EF0CB50EA; DTXSID5021419; (S)-a-Aminoindole-3-propionate; BDBM21974; HMS501G19; KBio1_000457; ZINC83315; TRYPTOPHAN [EP MONOGRAPH]; 3-(1H-indol-3-yl)-L-Alanine; L-a-Amino-3-indolepropionic acid; NINDS_000457; alpha-Aminoindole-3-propionic acid; HMS3263N07; Pharmakon1600-01500600; TRYPTOPHAN [USP MONOGRAPH]; TRYPTOPHAN, L- [WHO-DD]; ACT08662; HY-N0623; STR02722; (S)-alpha-Aminoindole-3-propionate; Tox21_201246; Tox21_300359; Tox21_501183; NSC757373; s3987; (s)-alpha-amino-beta-indolepropionate; L-Tryptophan, Vetec(TM), 98.5%; (S)-a-Amino-1H-indole-3-propanoate; AKOS015854052; Indoe-3-propionic acid, alpha-amino-; AM82273; CCG-205257; CS-W020011; DB00150; LP01183; NSC-757373; SDCCGSBI-0051150.P002; IDI1_000457; NCGC00015994-01; NCGC00094437-01; NCGC00094437-02; NCGC00094437-03; NCGC00094437-04; NCGC00094437-08; NCGC00254424-01; NCGC00258798-01; NCGC00261868-01; (S)-alpha-Amino-1H-indole-3-propanoate; AC-17050; BP-13286; SMR000326686; TS-04426; DB-029986; L-Tryptophan, BioUltra, >=99.5% (NT); EU-0101183; T0541; (S)-Tryptophan 1H-Indole-3-alanine, (S)-; EN300-52634; 73T223; C00078; D00020; L-.ALPHA.-AMINO-3-INDOLEPROPIONIC ACID; L-Tryptophan, reagent grade, >=98% (HPLC); M02943; P16427; AB00373874_05; L-Tryptophan, Vetec(TM) reagent grade, >=98%; (S)-2-amino-3-(1H-Indol-3-yl)-propionic acid; A837752; L-Tryptophan, Cell Culture Reagent (H-L-Trp-OH); Q181003; SR-01000075590; 4-(3-METHOXYANILINO)-4-OXOBUT-2-ENOICACID; N-ACETYLTRYPTOPHAN IMPURITY A [EP IMPURITY]; SR-01000075590-1; F0001-2364; Z756440056; 1H-INDOLE-3-PROPANOIC ACID, .ALPHA.-AMINO-, (S)-; L-Tryptophan, certified reference material, TraceCERT(R); Tryptophan, European Pharmacopoeia (EP) Reference Standard; L-Tryptophan, United States Pharmacopeia (USP) Reference Standard; L-Tryptophan, from non-animal source, meets EP, JP, USP testing specifications, suitable for cell culture, 99.0-101.0%
|