NPs Basic Information
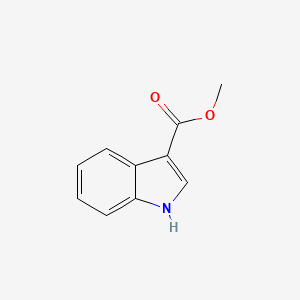
|
Name |
Methyl indole-3-carboxylate
|
| Molecular Formula | C10H9NO2 | |
| IUPAC Name* |
methyl 1H-indole-3-carboxylate
|
|
| SMILES |
COC(=O)C1=CNC2=CC=CC=C21
|
|
| InChI |
InChI=1S/C10H9NO2/c1-13-10(12)8-6-11-9-5-3-2-4-7(8)9/h2-6,11H,1H3
|
|
| InChIKey |
QXAUTQFAWKKNLM-UHFFFAOYSA-N
|
|
| Synonyms |
Methyl indole-3-carboxylate; 942-24-5; Methyl 1H-indole-3-carboxylate; Methyl 3-indolecarboxylate; Indole-3-carboxylic Acid Methyl Ester; 1H-Indole-3-carboxylic acid, methyl ester; 3-carbomethoxyindole; 3-methoxycarbonylindole; methylindole-3-carboxylate; 1H-Indole-3-carboxylic acid methyl ester; CHEBI:65019; Indole-3-carboxylic acid, methyl ester; CHEMBL2270066; MFCD00189407; 3-carbomethoxy indole; methyl indole 3-carboxylate; 3-methoxycarbonyl-1H-indole; SCHEMBL1093530; ZINC66126; DTXSID10343334; Indole-3-carboxylicacidmethylester; HMS1661G01; Methyl 1H-indole-3-carboxylate #; Methyl indole-3-carboxylate, 99%; AMY23351; BCP00917; CS-D1229; AB9732; BDBM50250885; METHYL INDOLE-3- CARBOXYLATE; STK397421; AKOS000579454; PB47482; SDCCGMLS-0065824.P001; HY-79635; SY020043; FT-0628332; I0491; Indole-3-carboxylic acid methyl ester, 99%; EN300-18395; I-2505; 7T-1502; Q27133581; Z57164966; F2190-0648; 3-Methoxycarbonylindole, 3-Carbomethoxyindole, Methyl indolyl-3-carboxylate
|
|
| CAS | 942-24-5 | |
| PubChem CID | 589098 | |
| ChEMBL ID | CHEMBL2270066 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 175.18 | ALogp: | 2.6 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 42.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 13 | QED Weighted: | 0.677 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.521 | MDCK Permeability: | 0.00001580 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.128 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.337 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.738 | Plasma Protein Binding (PPB): | 77.30% |
| Volume Distribution (VD): | 1.069 | Fu: | 22.44% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.989 | CYP1A2-substrate: | 0.855 |
| CYP2C19-inhibitor: | 0.913 | CYP2C19-substrate: | 0.334 |
| CYP2C9-inhibitor: | 0.394 | CYP2C9-substrate: | 0.897 |
| CYP2D6-inhibitor: | 0.449 | CYP2D6-substrate: | 0.755 |
| CYP3A4-inhibitor: | 0.457 | CYP3A4-substrate: | 0.2 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.205 | Half-life (T1/2): | 0.823 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.419 |
| Drug-inuced Liver Injury (DILI): | 0.686 | AMES Toxicity: | 0.015 |
| Rat Oral Acute Toxicity: | 0.494 | Maximum Recommended Daily Dose: | 0.344 |
| Skin Sensitization: | 0.573 | Carcinogencity: | 0.096 |
| Eye Corrosion: | 0.035 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.239 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005757 | 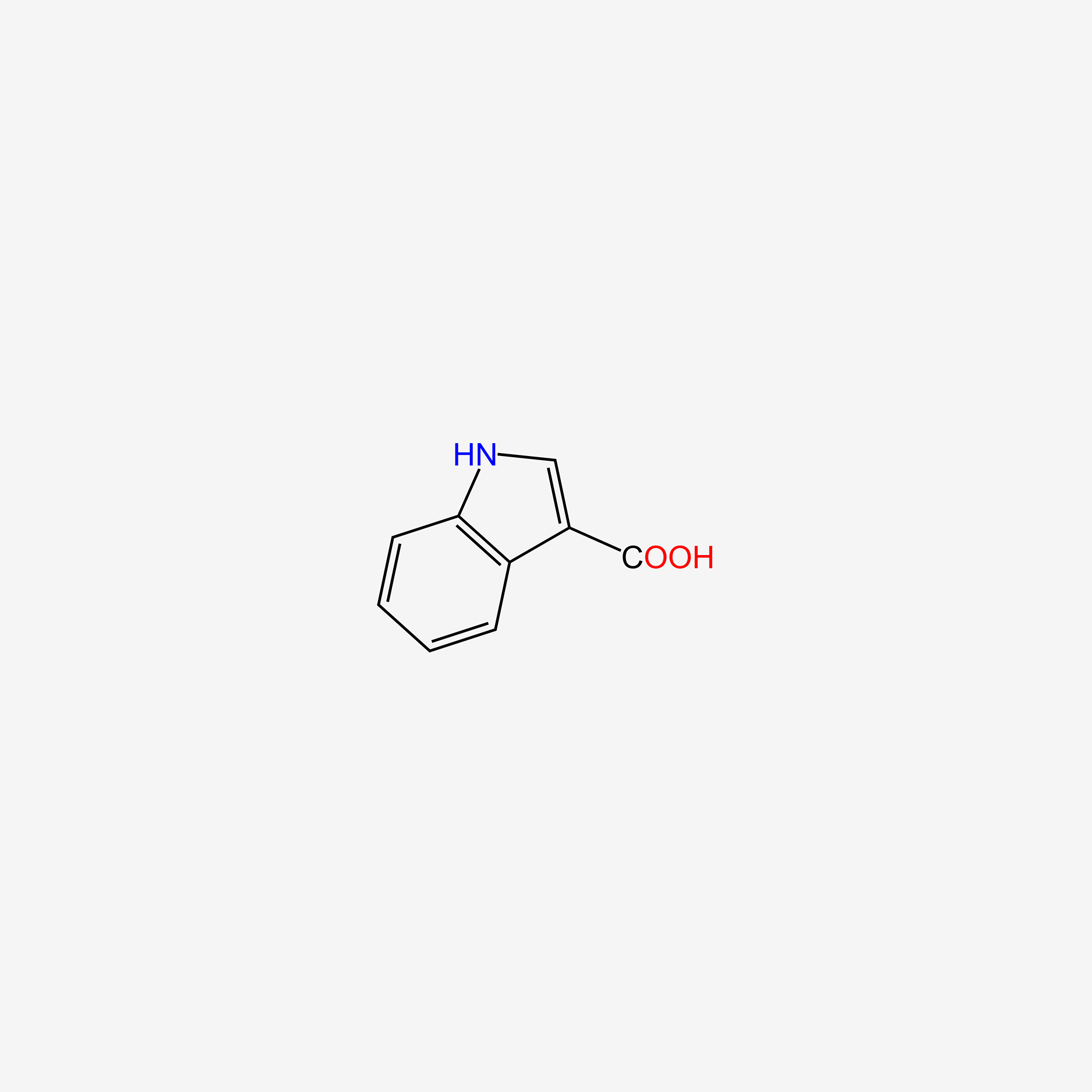 |
0.690 | D0K0KH | 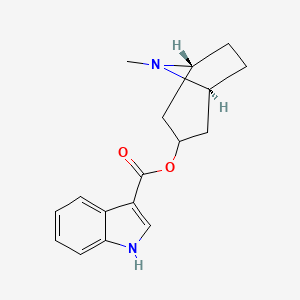 |
0.508 | ||
| ENC000999 | 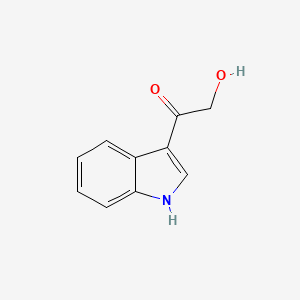 |
0.644 | D05EJG | 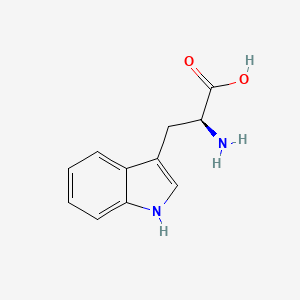 |
0.491 | ||
| ENC001448 | 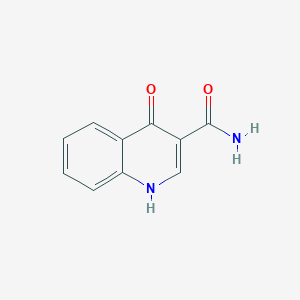 |
0.583 | D00YLW | 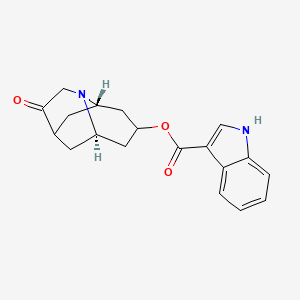 |
0.427 | ||
| ENC004871 | 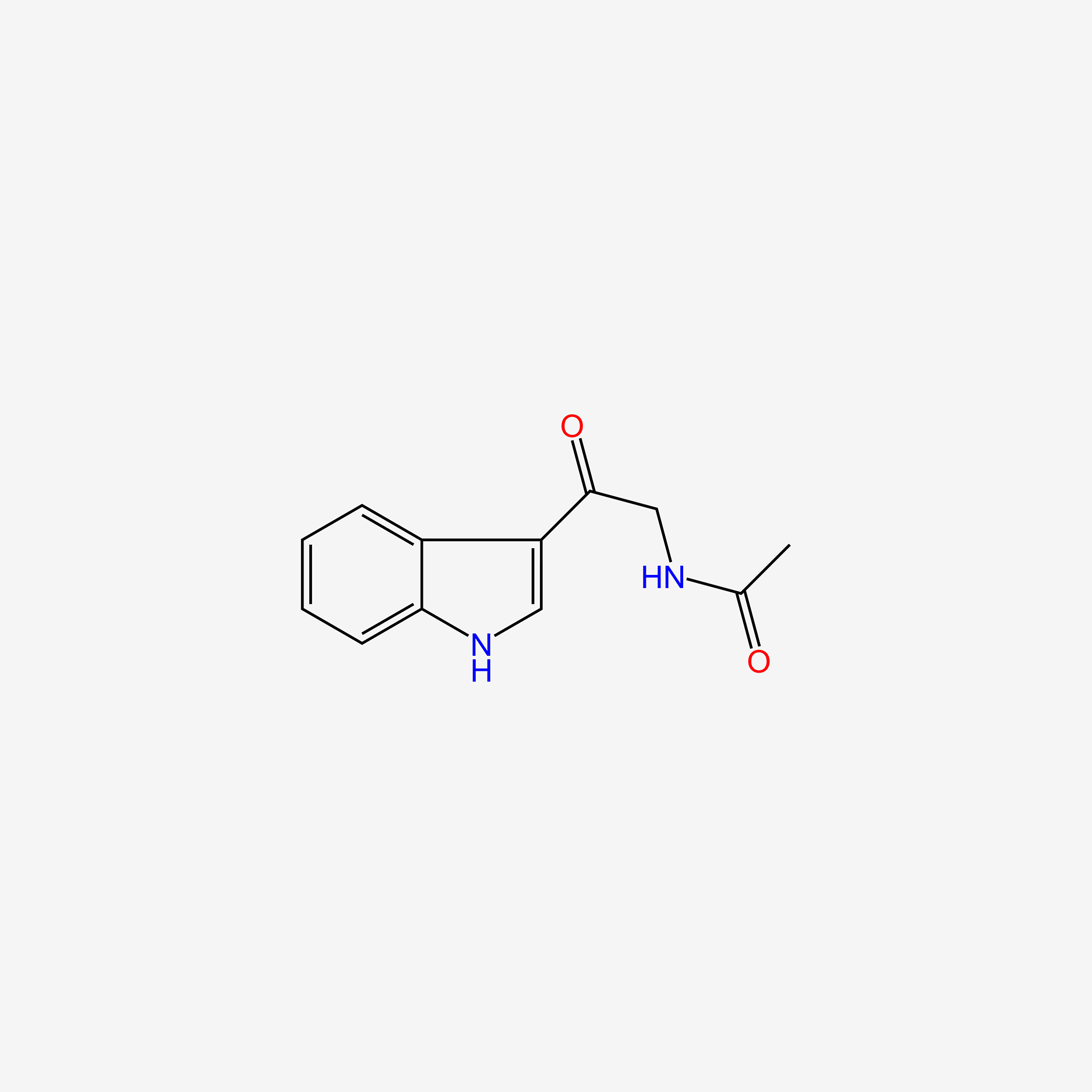 |
0.577 | D0AN7B | 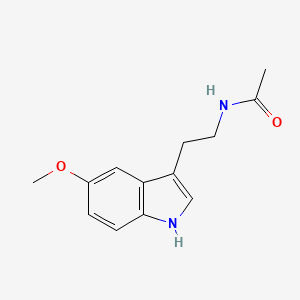 |
0.349 | ||
| ENC000043 | 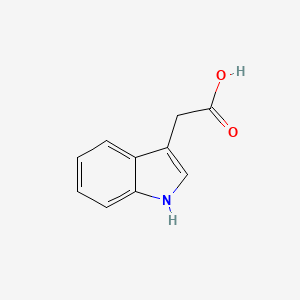 |
0.542 | D0W7WC | 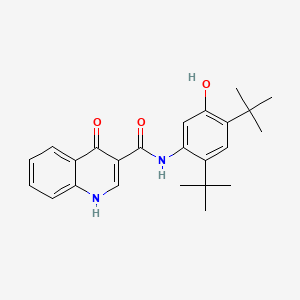 |
0.337 | ||
| ENC000341 |  |
0.533 | D0GY5Z | 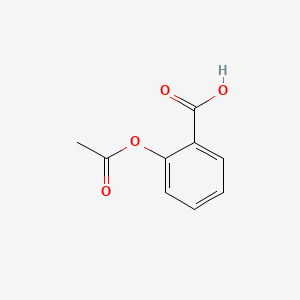 |
0.333 | ||
| ENC000104 | 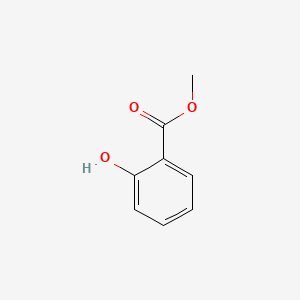 |
0.523 | D07HBX |  |
0.333 | ||
| ENC000303 | 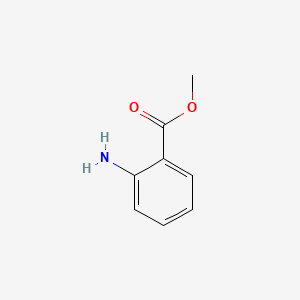 |
0.523 | D01JGV | 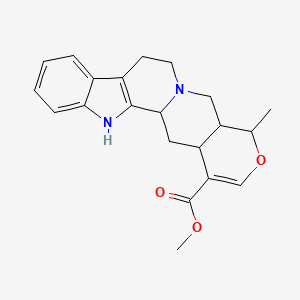 |
0.333 | ||
| ENC000694 | 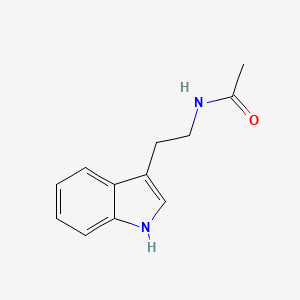 |
0.509 | D0U7GP | 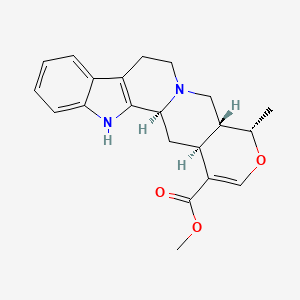 |
0.333 | ||
| ENC005609 | 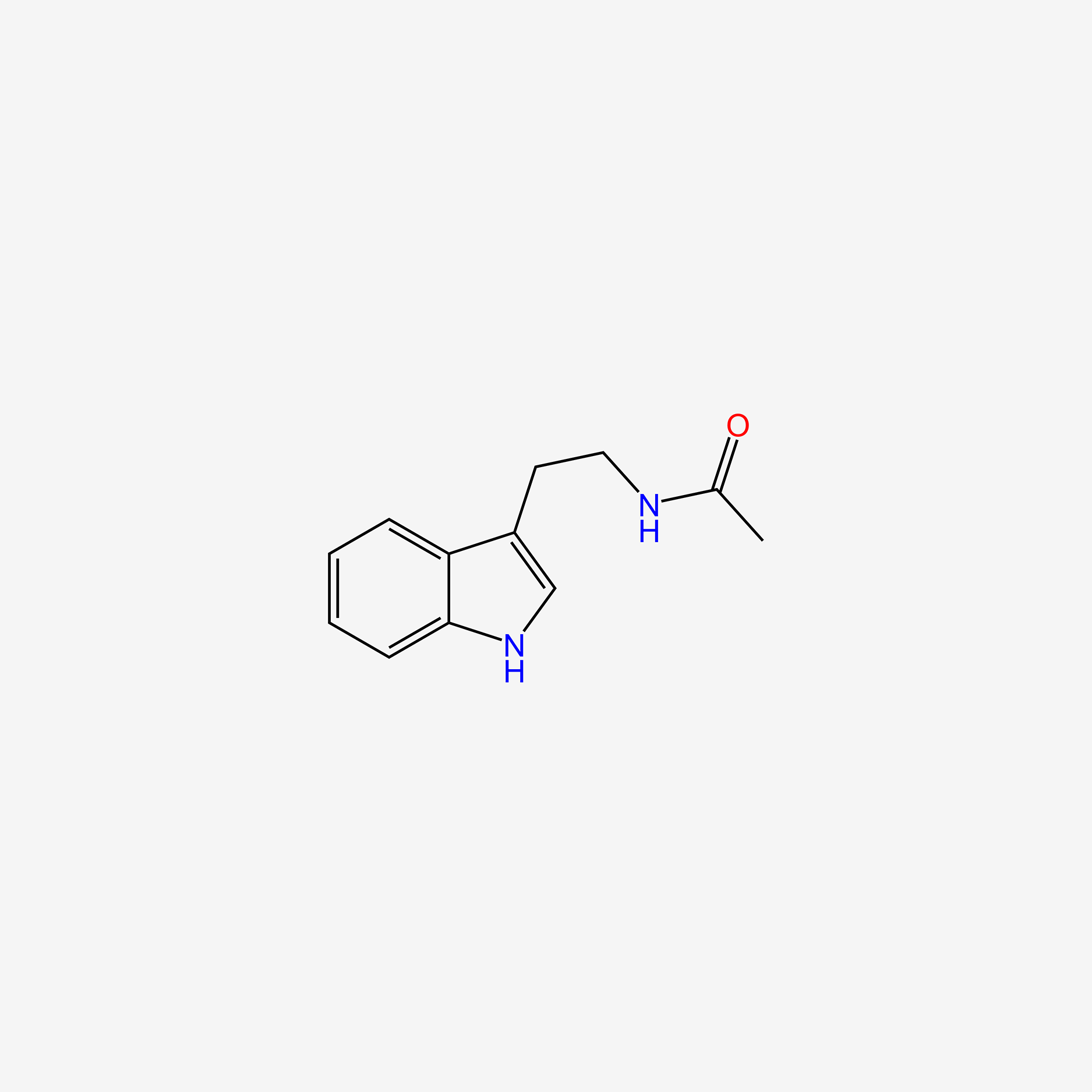 |
0.509 | D0X9RY |  |
0.319 | ||