NPs Basic Information
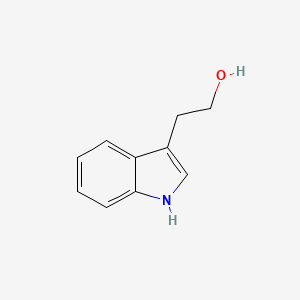
|
Name |
Tryptophol
|
| Molecular Formula | C10H11NO | |
| IUPAC Name* |
2-(1H-indol-3-yl)ethanol
|
|
| SMILES |
C1=CC=C2C(=C1)C(=CN2)CCO
|
|
| InChI |
InChI=1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
|
|
| InChIKey |
MBBOMCVGYCRMEA-UHFFFAOYSA-N
|
|
| Synonyms |
Tryptophol; 526-55-6; Indole-3-ethanol; 3-(2-Hydroxyethyl)indole; 1H-Indole-3-ethanol; 2-(1H-Indol-3-yl)ethanol; 3-Indoleethanol; 3-Indolylethanol; Indoleethanol; 2-(1h-indol-3-yl)ethan-1-ol; Indole ethanol; 2-(3-Indolyl)ethanol; Ethanol, 2-indol-3-yl-; ETHANOL, 3-INDOLYL-; 1H-Indolyl-3-ethanol; beta-Indol-3-ylethanol; 3-(beta-Hydroxyethyl)indole; 2-(1H-Indol-3-yl)-ethanol; NSC 3884; IEA; 2-(indol-3-yl)ethanol; Maybridge1_002422; .beta.-(3-Indole)ethanol; 3-(.beta.-Hydroxyethyl)indole; CHEBI:17890; 5809LZ7G1U; NSC-3884; MFCD00005659; EINECS 208-393-2; BRN 0125553; UNII-5809LZ7G1U; 3-indolethanol; Tryptaphol, 6; ZCW; (indol-3-yl)ethanol; 2-(3-Indolylethanol; b-(3-Indole)ethanol; TRYPTOPHOL [MI]; beta-(3-Indole)ethanol; 3-(b-Hydroxyethyl)indole; 2-(indol-3-yl)-ethanol; bmse000473; 3-(2-Hydroxyethyl) Indole; 5-21-03-00061 (Beilstein Handbook Reference); MLS001250154; DivK1c_001174; SCHEMBL196126; 2-(3-INDOLE)ETHANOL; WLN: T56 BMJ D2Q; 2-INDOLYL(3)-ETHANOL; CHEMBL226545; ISUPSL100239; ZINC3252; DTXSID2060173; BDBM92686; HMS548G02; MBBOMCVGYCRMEA-UHFFFAOYSA-; 2-(1H-Indol-3-yl)ethanol #; NSC3884; 3-(2-Hydroxy-ethyl)-1H-indole; 3-(2-Hydroxyethyl)indole, 97%; HMS2270O23; .BETA.-INDOLYLETHYL ALCOHOL; 3-.BETA.-HYDROXYETHYLINDOLE; BCP15412; 2-(3-INDOLYL)ETHYL ALCOHOL; 3-.OMEGA.-HYDROXYETHYLINDOLE; AM1052; BBL027534; s4858; STL382051; 2-(1H-indol-3-yl)ethanol;Tryptophol; AKOS002666320; AC-3286; CCG-266289; CS-W010871; HY-W010155; SB14961; CDS1_000134; NCGC00247328-01; AS-15780; SMR000686021; SY004666; DB-007578; FT-0600657; I0030; EN300-70080; C00955; T-8495; T-8500; 526T556; A829187; AE-508/40182784; Q5479351; Z1123806416; 3-(2-Hydroxyethyl)indole, Vetec(TM) reagent grade, 97%; 08DD2DF3-02E7-44DC-9714-864651873577
|
|
| CAS | 526-55-6 | |
| PubChem CID | 10685 | |
| ChEMBL ID | CHEMBL226545 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 161.2 | ALogp: | 1.8 |
| HBD: | 2 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 36.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.696 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.381 | MDCK Permeability: | 0.00001480 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.026 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.716 |
| 30% Bioavailability (F30%): | 0.987 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.834 | Plasma Protein Binding (PPB): | 57.27% |
| Volume Distribution (VD): | 1.96 | Fu: | 32.15% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.97 | CYP1A2-substrate: | 0.815 |
| CYP2C19-inhibitor: | 0.64 | CYP2C19-substrate: | 0.243 |
| CYP2C9-inhibitor: | 0.065 | CYP2C9-substrate: | 0.921 |
| CYP2D6-inhibitor: | 0.412 | CYP2D6-substrate: | 0.842 |
| CYP3A4-inhibitor: | 0.142 | CYP3A4-substrate: | 0.223 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.564 | Half-life (T1/2): | 0.878 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.36 |
| Drug-inuced Liver Injury (DILI): | 0.193 | AMES Toxicity: | 0.04 |
| Rat Oral Acute Toxicity: | 0.432 | Maximum Recommended Daily Dose: | 0.249 |
| Skin Sensitization: | 0.743 | Carcinogencity: | 0.151 |
| Eye Corrosion: | 0.331 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.405 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000043 | 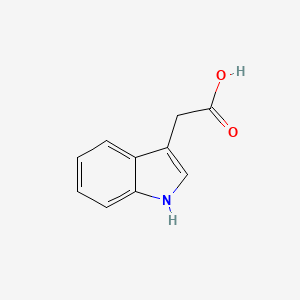 |
0.636 | D05EJG | 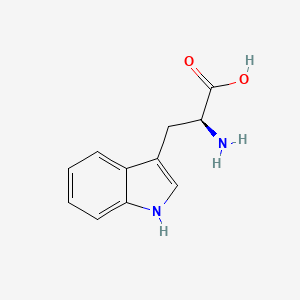 |
0.571 | ||
| ENC000042 | 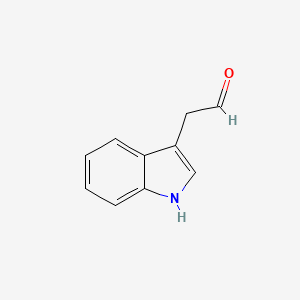 |
0.628 | D0AN7B | 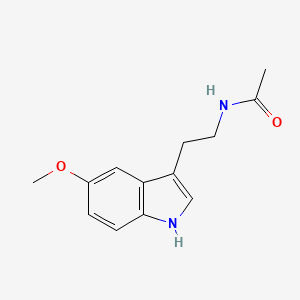 |
0.361 | ||
| ENC005609 | 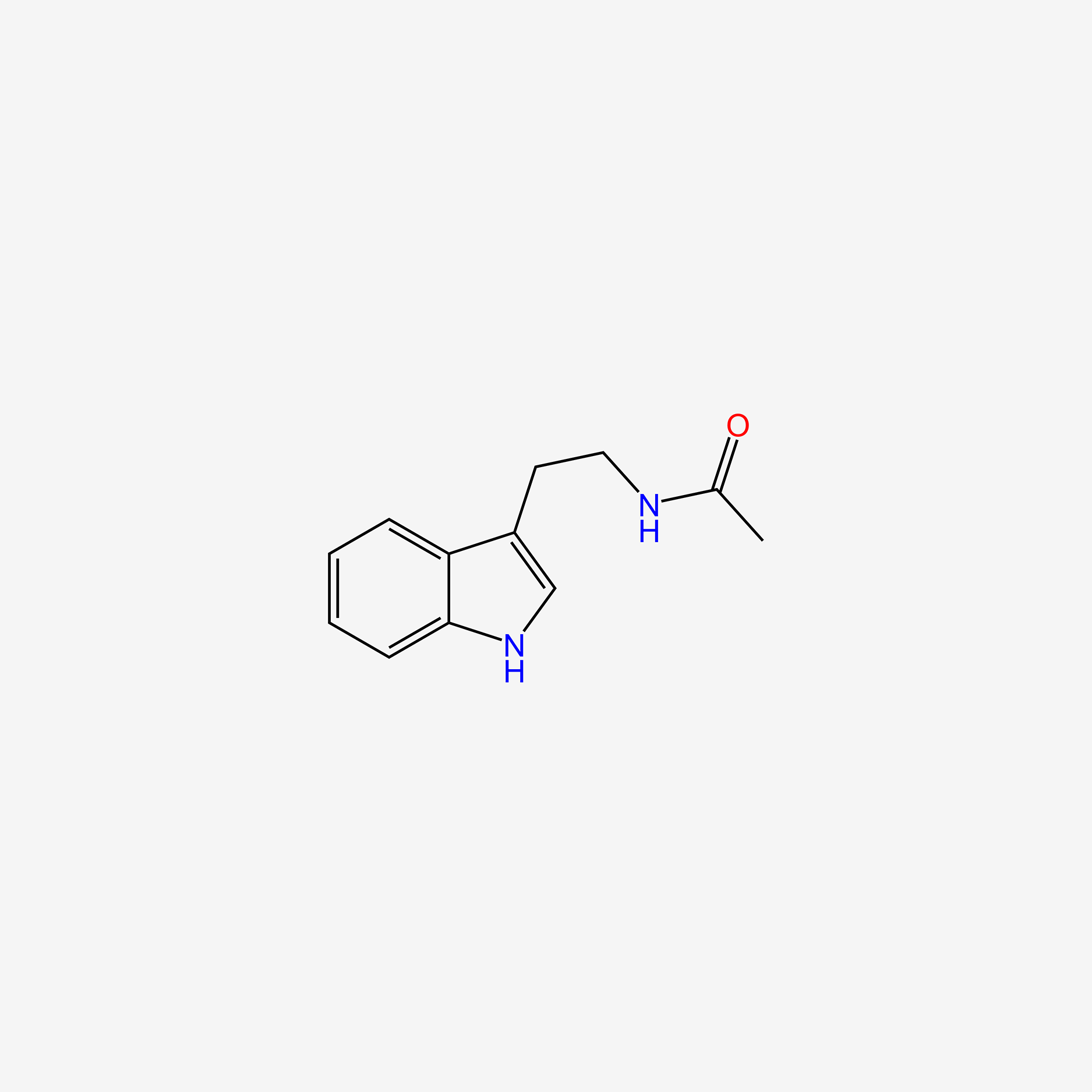 |
0.625 | D05OIS |  |
0.349 | ||
| ENC005018 | 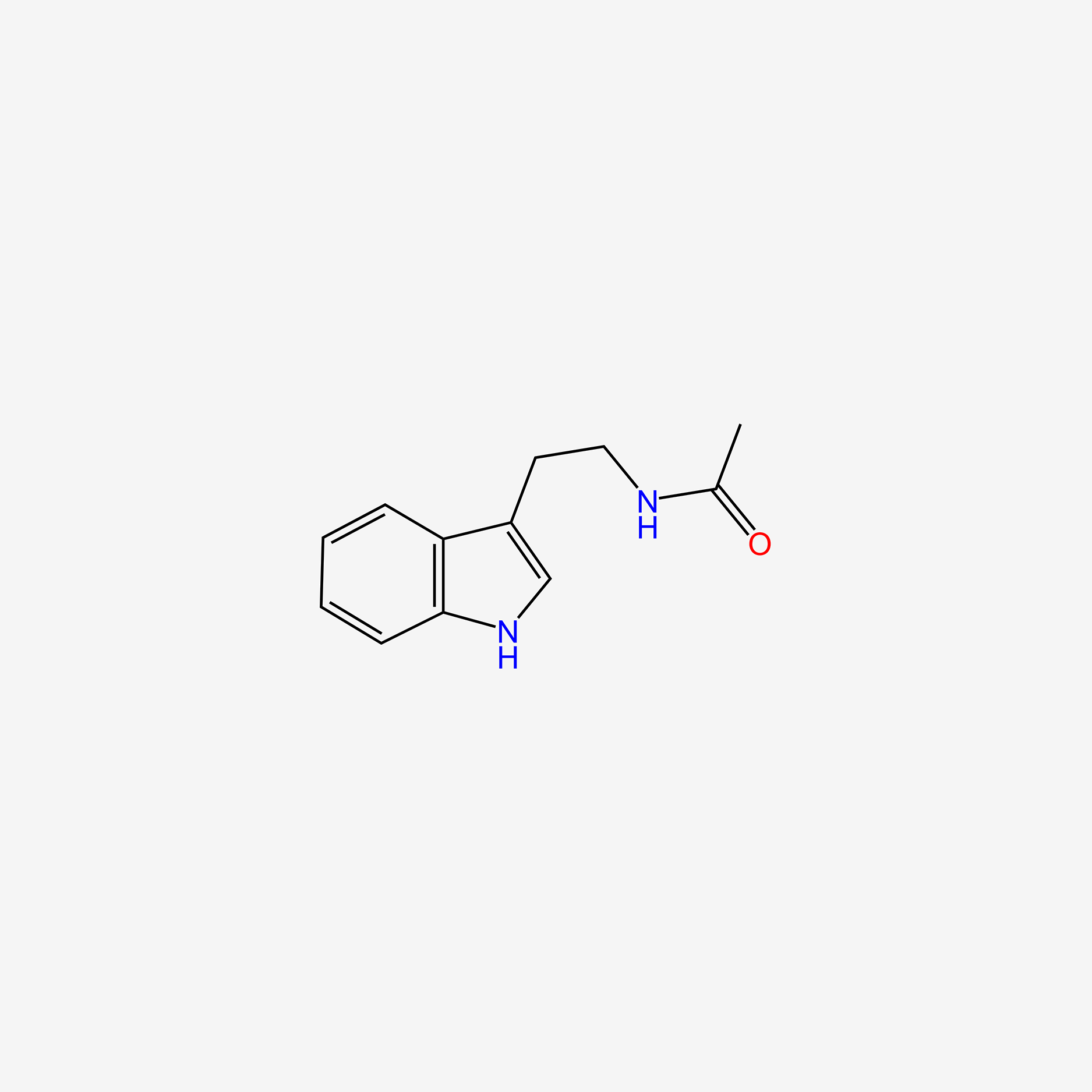 |
0.625 | D0S9MU | 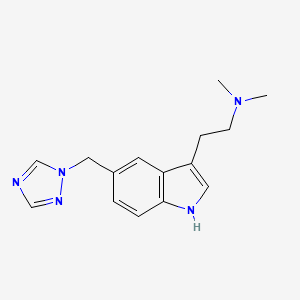 |
0.329 | ||
| ENC000694 | 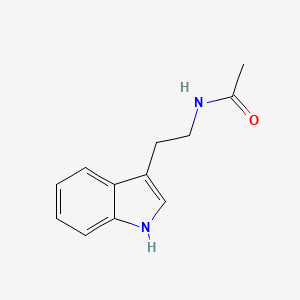 |
0.625 | D0Z6UC | 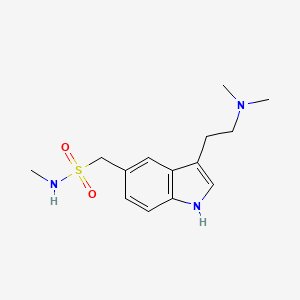 |
0.324 | ||
| ENC000140 | 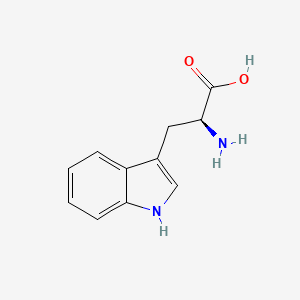 |
0.571 | D0K0KH | 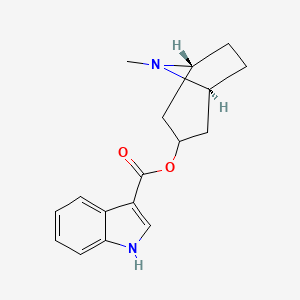 |
0.315 | ||
| ENC004706 | 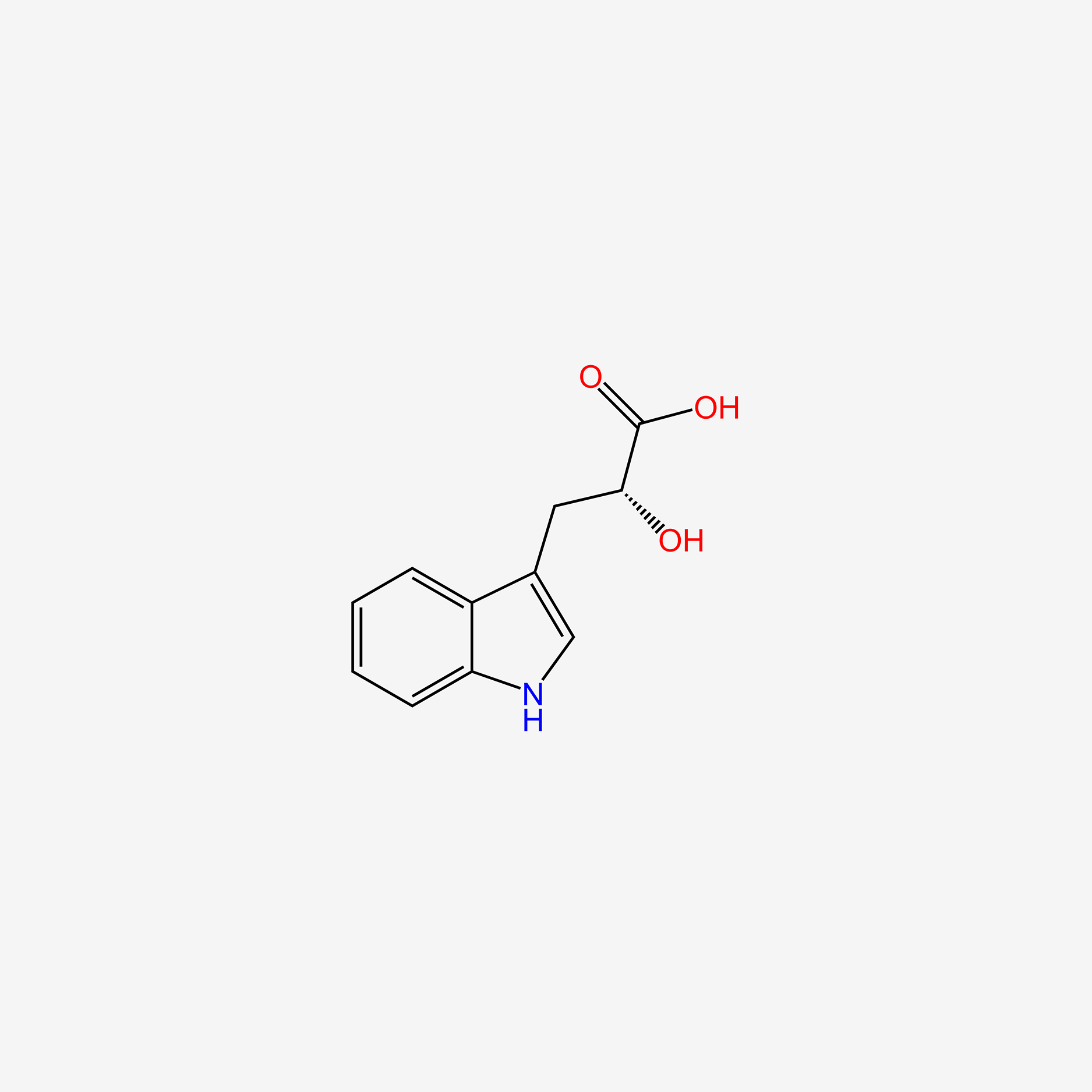 |
0.571 | D0P9AC | 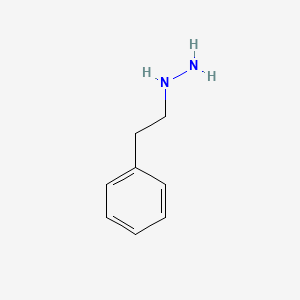 |
0.306 | ||
| ENC000999 | 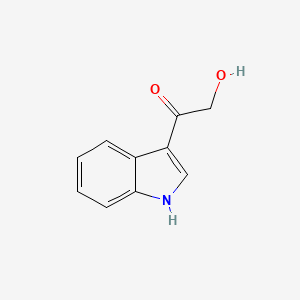 |
0.565 | D0F2PO | 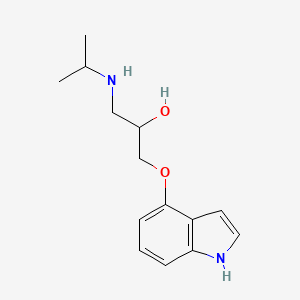 |
0.303 | ||
| ENC005757 | 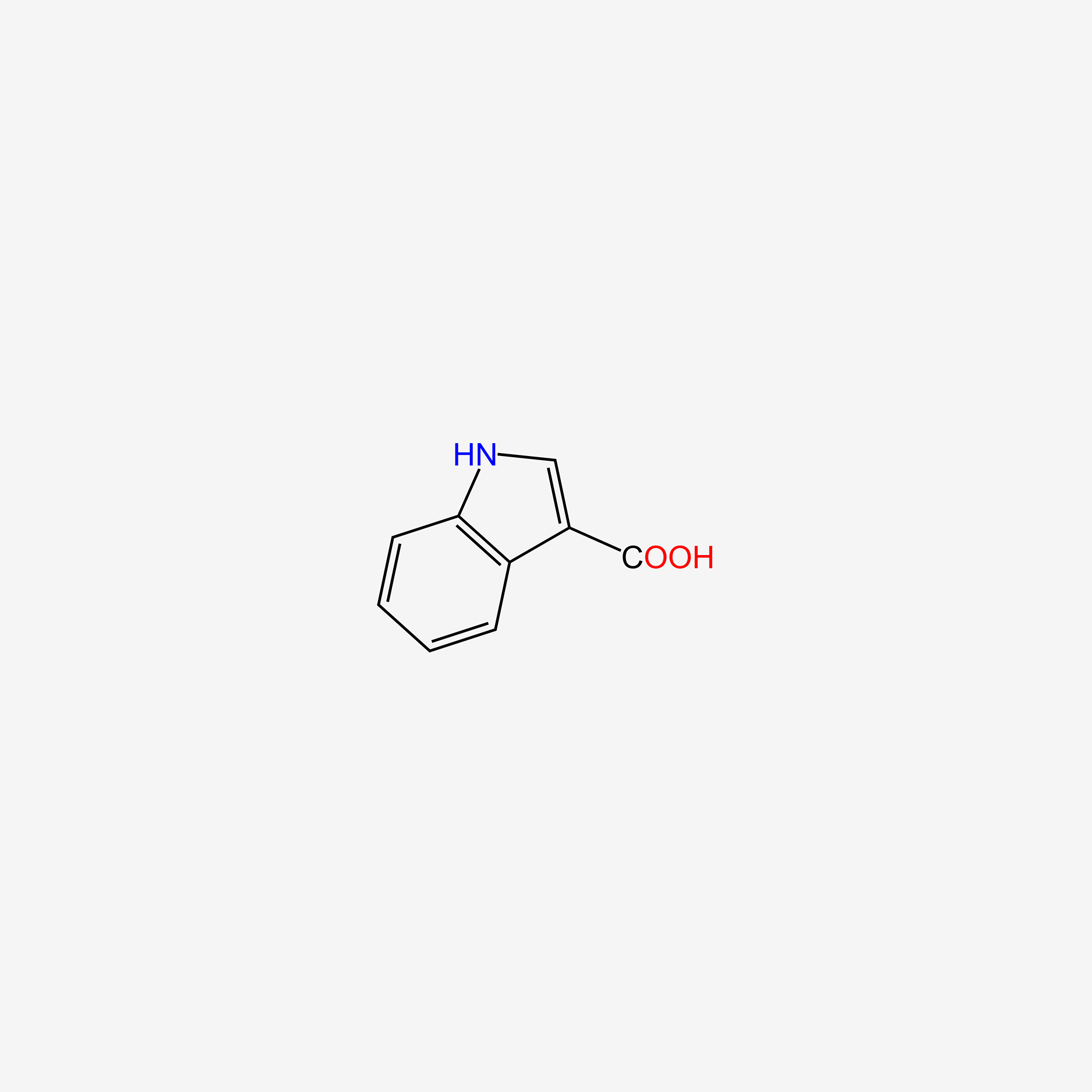 |
0.533 | D0NG7O | 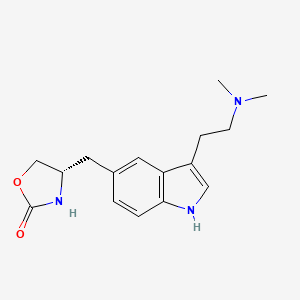 |
0.301 | ||
| ENC000341 |  |
0.523 | D02DMQ | 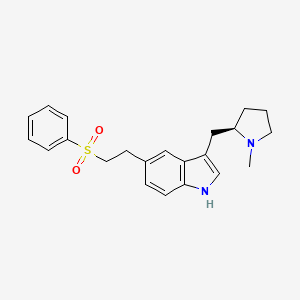 |
0.299 | ||