NPs Basic Information
|
Name |
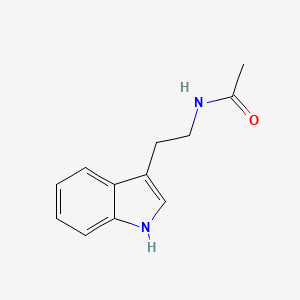
N-Acetyltryptamine
|
| Molecular Formula | C12H14N2O | |
| IUPAC Name* |
N-[2-(1H-indol-3-yl)ethyl]acetamide
|
|
| SMILES |
CC(=O)NCCC1=CNC2=CC=CC=C21
|
|
| InChI |
InChI=1S/C12H14N2O/c1-9(15)13-7-6-10-8-14-12-5-3-2-4-11(10)12/h2-5,8,14H,6-7H2,1H3,(H,13,15)
|
|
| InChIKey |
NVUGEQAEQJTCIX-UHFFFAOYSA-N
|
|
| Synonyms |
N-Acetyltryptamine; 1016-47-3; N-[2-(1H-Indol-3-yl)ethyl]acetamide; N-(2-(1H-indol-3-yl)ethyl)acetamide; Acetotryptamide; 3-(2-N-Acetylaminoethyl)indole; Acetamide, N-[2-(1H-indol-3-yl)ethyl]-; MFCD00209910; CHEMBL33171; N-[2-(1H-Indol-3-yl)-ethyl]-acetamide; CHEBI:55515; Acetamide, N-(2-(1H-indol-3-yl)ethyl)-; N-Acetyltryptamine; N10-Acetyltryptamine; Nb-Acetyltryptamine; Nomega-Acetyltryptamine; SMR000686036; SR-01000075685; Nb-acetyltryptamine; Acetamide, N-(2-indol-3-ylethyl)-; Acetyltryptamine, N-; Tocris-0357; Lopac-A-7342; N-Acetyltryptamine, powder; 3-(2-Acetamidoethyl)indole; Lopac0_000101; MLS001250169; MLS002153204; SCHEMBL468850; ISUPSL100255; ACon1_000465; DTXSID30144042; HMS2270O21; HMS3260E04; HMS3266I05; HMS3411G05; HMS3675G05; ZINC174849; BAA01647; Tox21_500101; BDBM50282758; PDSP1_001815; PDSP2_001798; STL352108; AKOS000639631; CCG-204196; LP00101; SDCCGSBI-0050089.P002; NCGC00015088-01; NCGC00015088-02; NCGC00015088-03; NCGC00015088-04; NCGC00015088-05; NCGC00015088-06; NCGC00015088-07; NCGC00015088-08; NCGC00015088-09; NCGC00024552-01; NCGC00024552-02; NCGC00024552-03; NCGC00024552-04; NCGC00024552-05; NCGC00024552-06; NCGC00260786-01; AS-63601; Acetamide,N-[2-(1H-indol-3-yl)ethyl]-; N-[2-(1H-Indol-3-yl)ethyl]acetamide #; HY-100908; CS-0020578; EU-0033445; EU-0100101; A 7342; EN300-189721; N17090; Acetamide, N-(2-indol-3-ylethyl)- (7CI,8CI); Acetotryptamide N-Acetyl-2-(indol-3-yl)ethylamine; Acetamide, N-[2-(1H-indol-3-yl)ethyl]- (9CI); J-000457; SR-01000075685-1; SR-01000075685-2; SR-01000075685-4; BRD-K73700643-001-04-7; BRD-K73700643-001-10-4; Q27124335; Z26395416; 2-(n,n-dimethyliminium)-4-ethyl-5-mercapto-1,3-dithiol,innersalt; NCGC00015088-09_C12H14N2O_N-[2-(1H-Indol-3-yl)ethyl]acetamide; 374572-55-1; 7AN
|
|
| CAS | 1016-47-3 | |
| PubChem CID | 70547 | |
| ChEMBL ID | CHEMBL33171 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 202.25 | ALogp: | 1.8 |
| HBD: | 2 | HBA: | 1 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 44.9 | Aromatic Rings: | 2 |
| Heavy Atoms: | 15 | QED Weighted: | 0.789 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.65 | MDCK Permeability: | 0.00001210 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.986 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.122 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.695 | Plasma Protein Binding (PPB): | 37.08% |
| Volume Distribution (VD): | 1.298 | Fu: | 44.49% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.948 | CYP1A2-substrate: | 0.882 |
| CYP2C19-inhibitor: | 0.688 | CYP2C19-substrate: | 0.434 |
| CYP2C9-inhibitor: | 0.075 | CYP2C9-substrate: | 0.851 |
| CYP2D6-inhibitor: | 0.482 | CYP2D6-substrate: | 0.857 |
| CYP3A4-inhibitor: | 0.179 | CYP3A4-substrate: | 0.177 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.732 | Half-life (T1/2): | 0.894 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.37 |
| Drug-inuced Liver Injury (DILI): | 0.289 | AMES Toxicity: | 0.117 |
| Rat Oral Acute Toxicity: | 0.254 | Maximum Recommended Daily Dose: | 0.78 |
| Skin Sensitization: | 0.344 | Carcinogencity: | 0.082 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.069 |
| Respiratory Toxicity: | 0.033 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005018 | 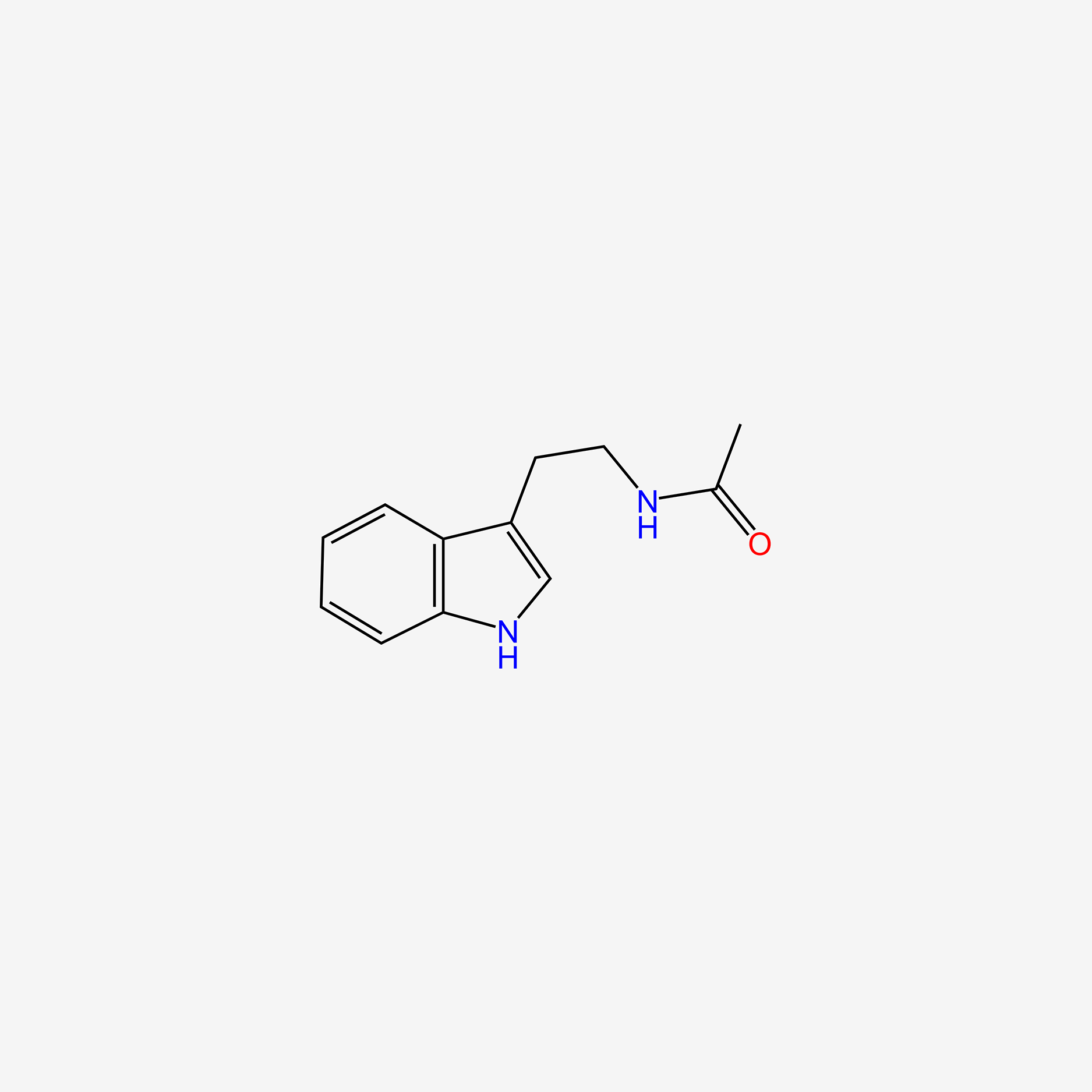 |
1.000 | D0AN7B | 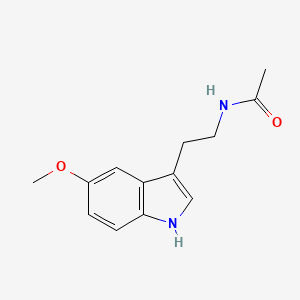 |
0.625 | ||
| ENC005609 | 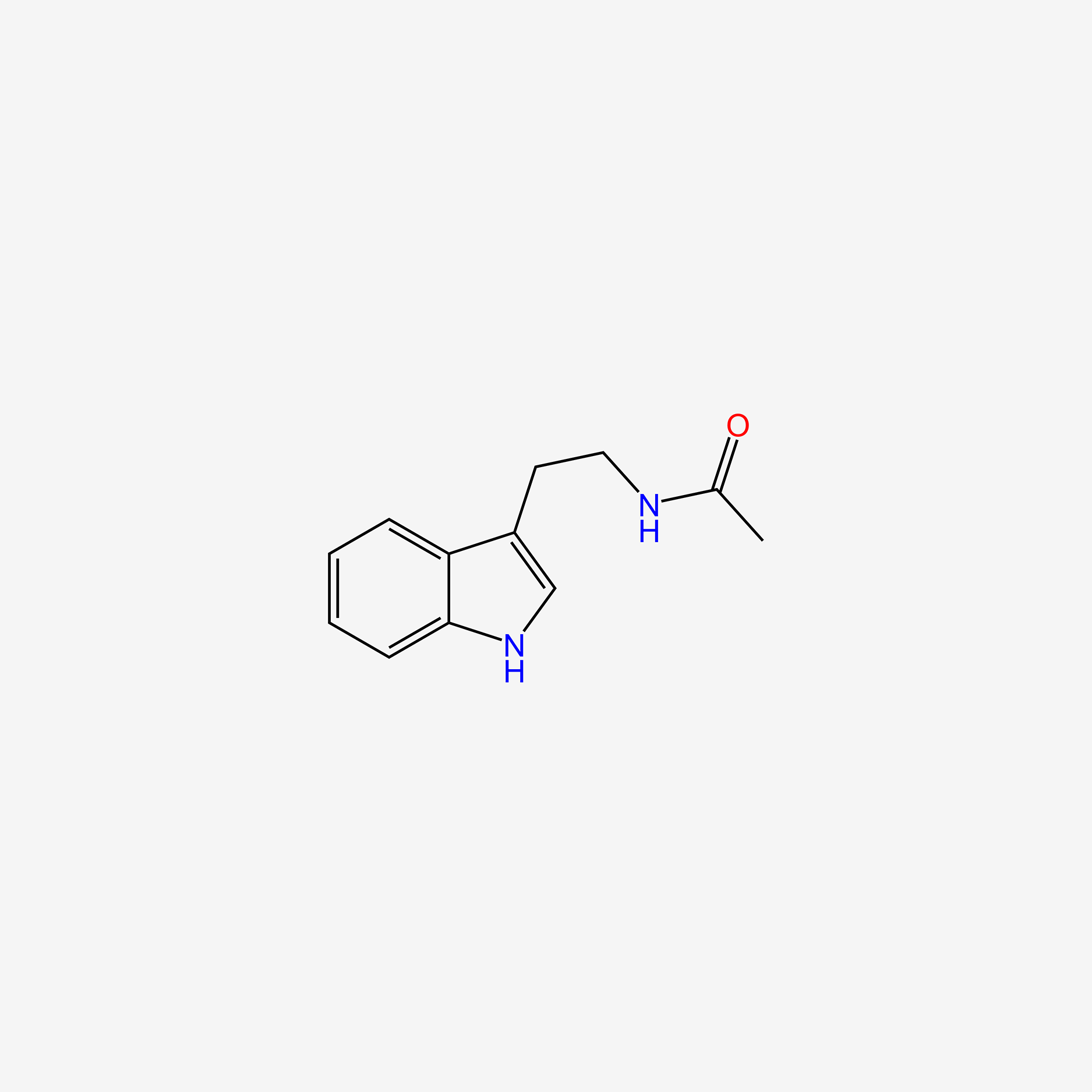 |
1.000 | D05EJG | 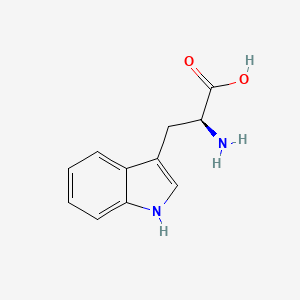 |
0.545 | ||
| ENC000363 | 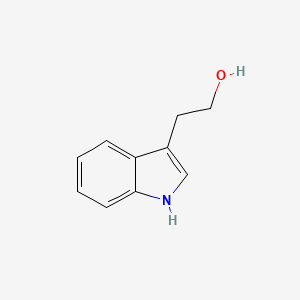 |
0.625 | D0K0KH | 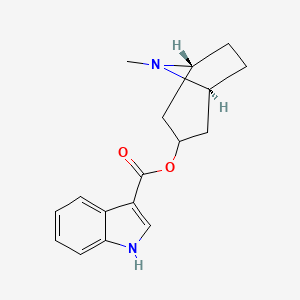 |
0.351 | ||
| ENC000043 | 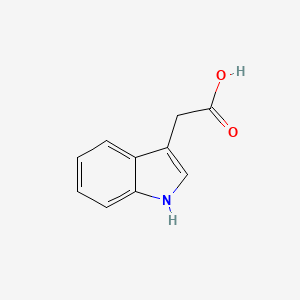 |
0.600 | D0Z6UC | 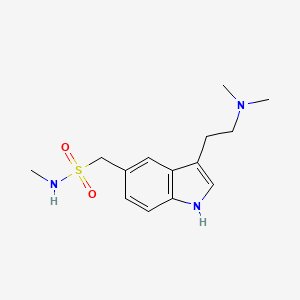 |
0.342 | ||
| ENC004871 | 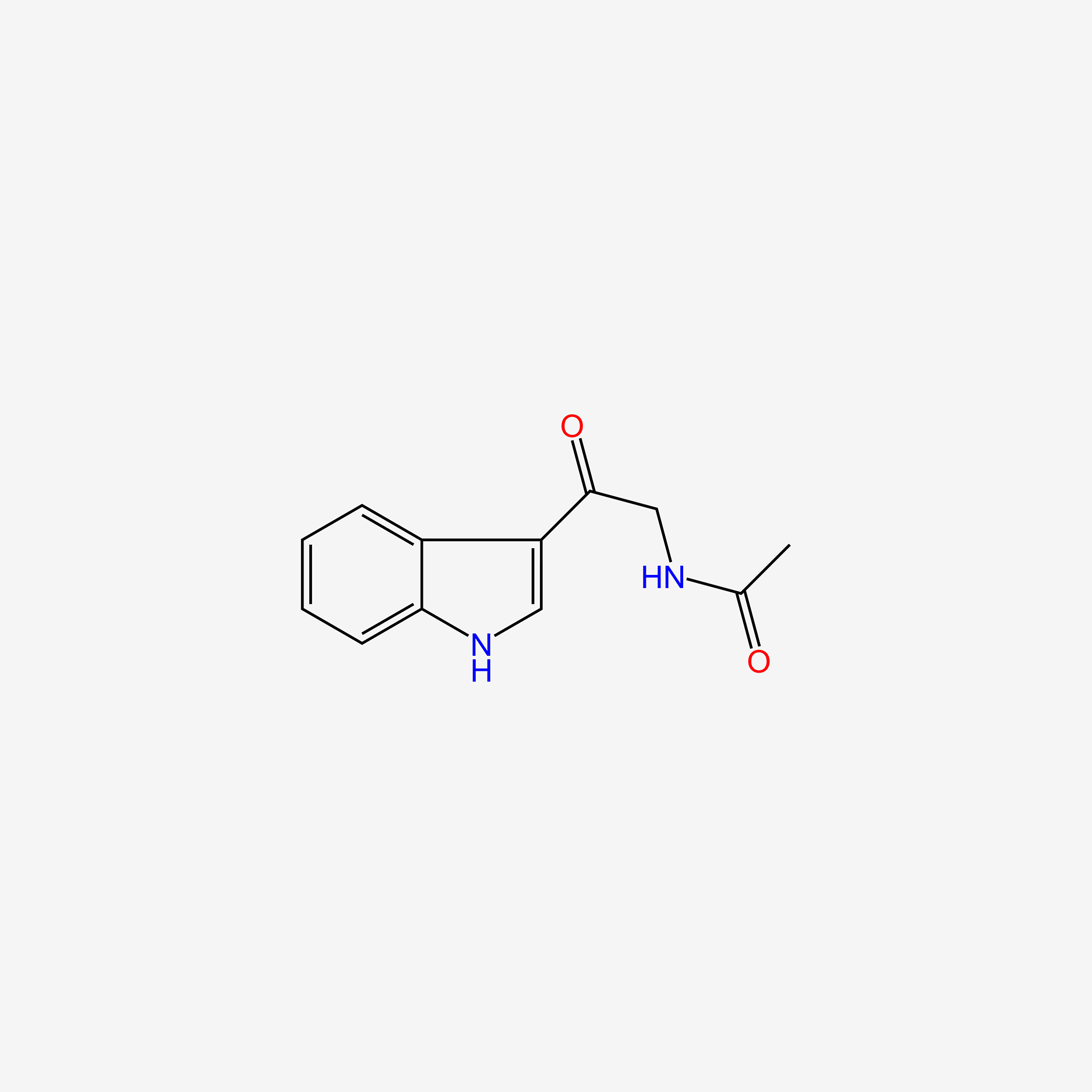 |
0.600 | D0S9MU | 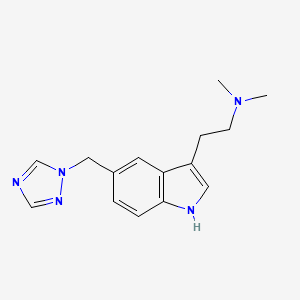 |
0.312 | ||
| ENC000042 | 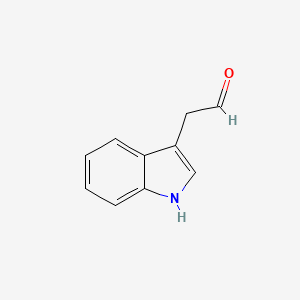 |
0.560 | D00DZN |  |
0.311 | ||
| ENC004706 | 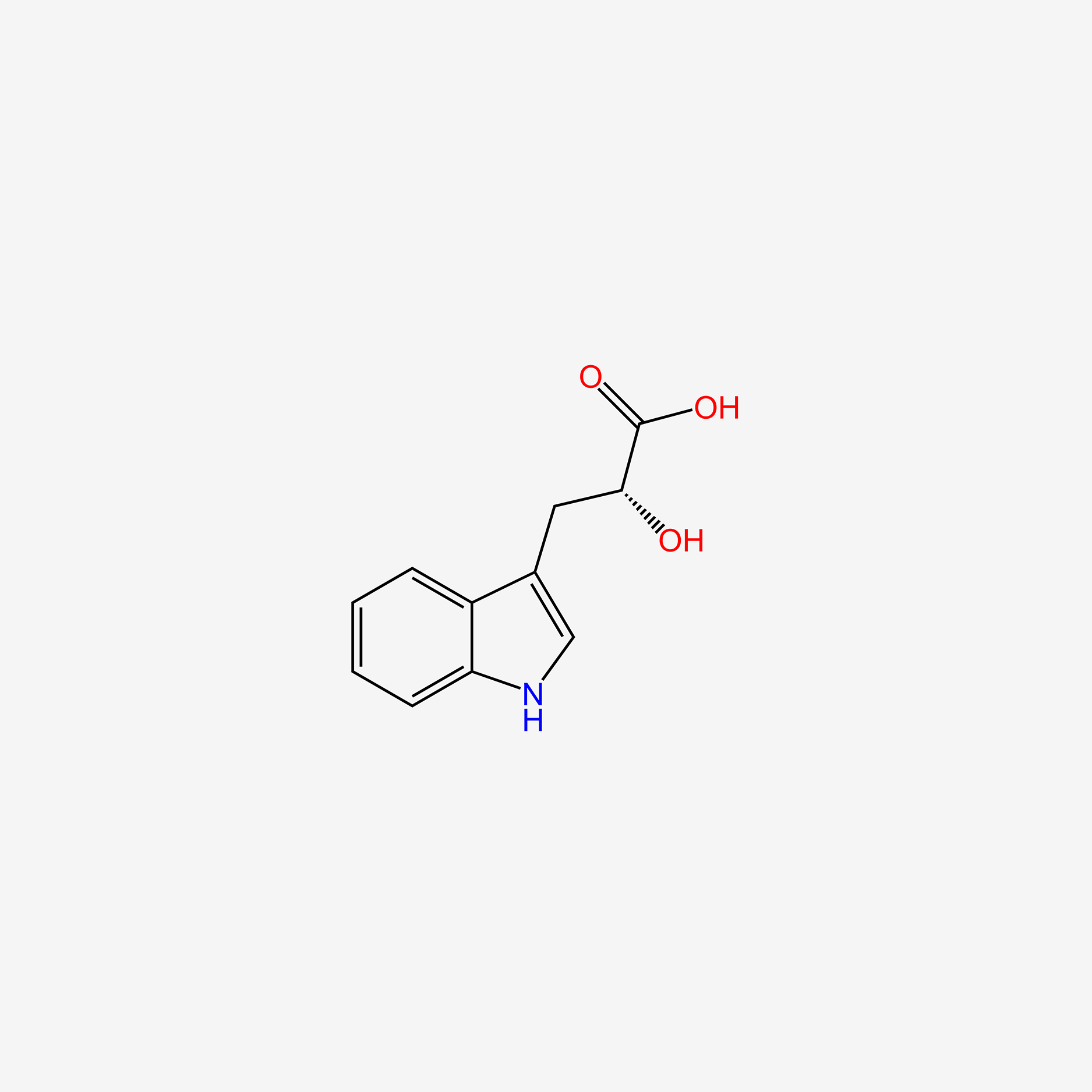 |
0.545 | D0E3SH | 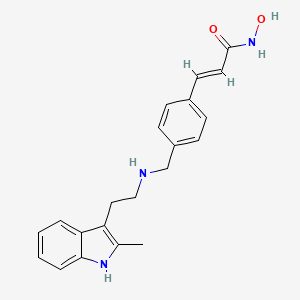 |
0.311 | ||
| ENC000140 | 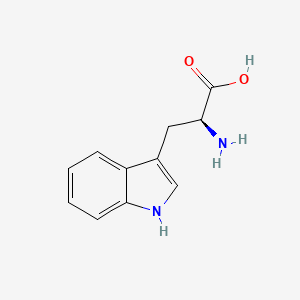 |
0.545 | D05OFX | 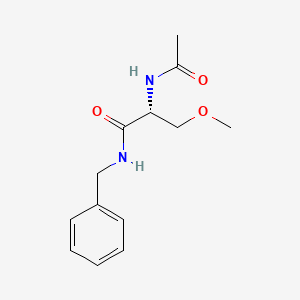 |
0.310 | ||
| ENC000693 | 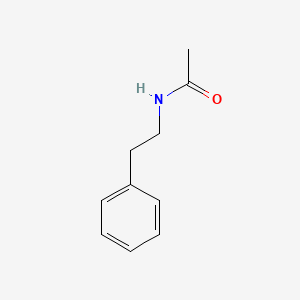 |
0.540 | D0P9AC | 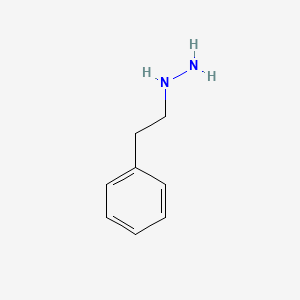 |
0.309 | ||
| ENC005757 | 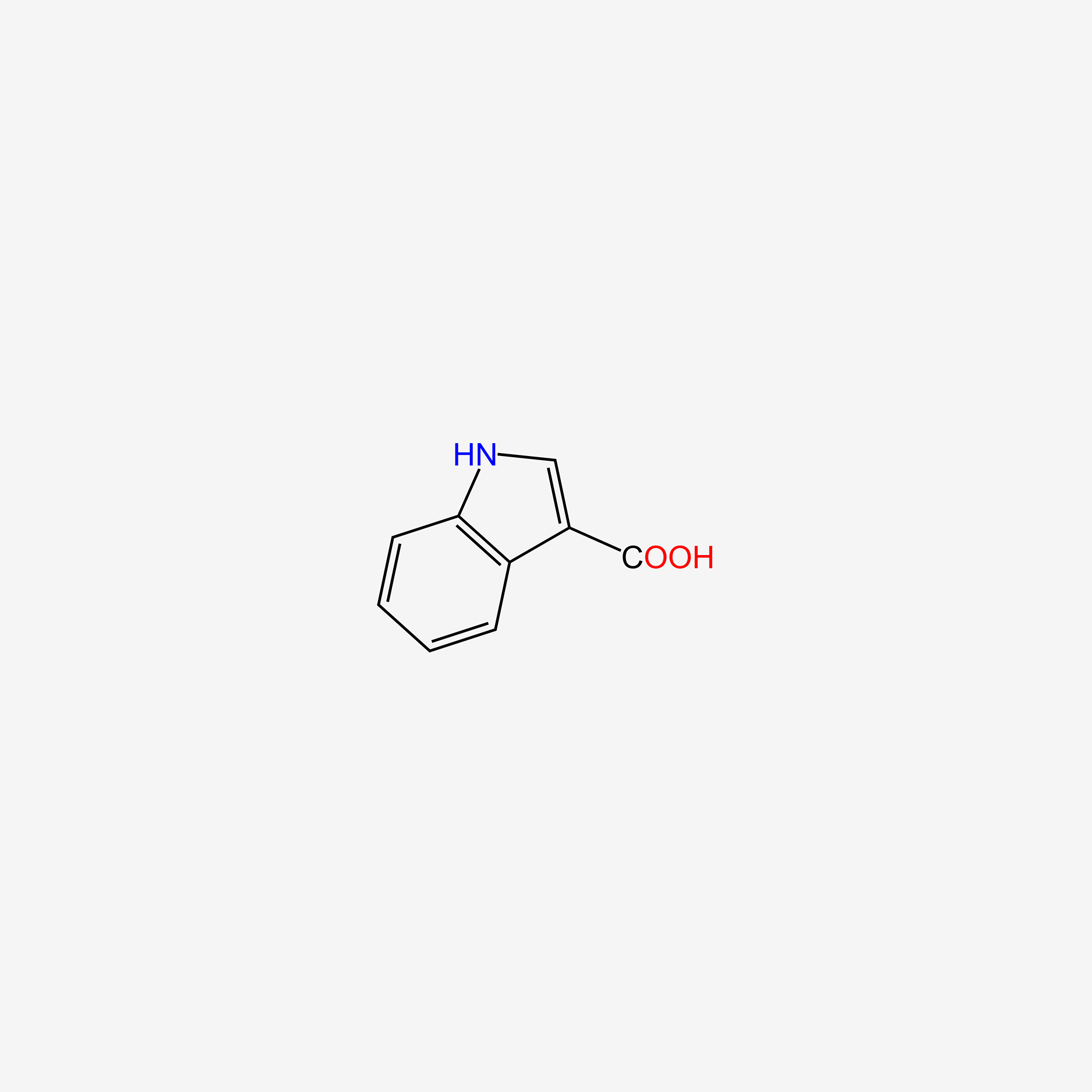 |
0.510 | D0NG7O | 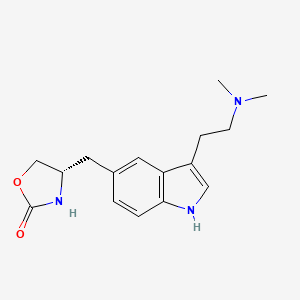 |
0.304 | ||