NPs Basic Information
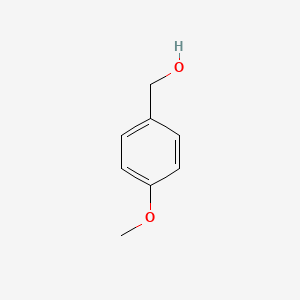
|
Name |
4-Methoxybenzyl alcohol
|
| Molecular Formula | C8H10O2 | |
| IUPAC Name* |
(4-methoxyphenyl)methanol
|
|
| SMILES |
COC1=CC=C(C=C1)CO
|
|
| InChI |
InChI=1S/C8H10O2/c1-10-8-4-2-7(6-9)3-5-8/h2-5,9H,6H2,1H3
|
|
| InChIKey |
MSHFRERJPWKJFX-UHFFFAOYSA-N
|
|
| Synonyms |
4-METHOXYBENZYL ALCOHOL; (4-Methoxyphenyl)methanol; 105-13-5; Anise alcohol; Anisyl alcohol; p-Methoxybenzyl alcohol; p-Anisyl alcohol; Benzenemethanol, 4-methoxy-; Anisic alcohol; p-Anisol alcohol; 4-Methoxybenzenemethanol; Benzyl alcohol, p-methoxy-; Anis alcohol; 4-Methoxybenzylalcohol; FEMA No. 2099; 4-Anisylalcohol; NSC 2151; p-methoxy-benzyl alcohol; MFCD00004653; (4-methoxyphenyl)-methanol; (4-Methoxy-phenyl)-methanol; 7N6XGV3U49; BENZENEMETHANOL,AR-METHOXY-; NSC-2151; 4-METHOXY-[7-13C]-BENZYL ALCOHOL; Anisalcohol, p-; DSSTox_CID_24357; [4-(methyloxy)phenyl]methanol; Anisyl alcohol (natural); (4-(METHYLOXY)PHENYL)METHANOL; CCRIS 5111; EINECS 203-273-6; METHOXYBENZYLALCOHOL; BRN 0636654; UNII-7N6XGV3U49; Anisalkohol; p-anisalcohol; AI3-01170; para-anisyl alcohol; JandaJel(TM)-Wang; p-methoxybenzylalcohol; 4-methoxyphenylmethanol; 4-methoxy-benzylalcohol; 4-methoxylbenzyl alcohol; 4-methyoxybenzyl alcohol; 4-methoxy-benzenemethanol; 4-methoxy-benzyl alcohol; 4-(Hydroxymethyl)anisole; para-methoxybenzyl alcohol; bmse010025; EC 203-273-6; ANISE ALCOHOL [MI]; DSSTox_RID_80166; DSSTox_RID_82376; 4-methoxyphenylmethyl alcohol; DSSTox_GSID_44357; DSSTox_GSID_47486; SCHEMBL27329; WLN: Q1R DO1; (4-Methoxyphenyl)methanol #; ANISE ALCOHOL [INCI]; ANISYL ALCOHOL [FCC]; 4-06-00-05909 (Beilstein Handbook Reference); ANISYL ALCOHOL [FHFI]; 4-Methoxybenzyl alcohol, 98%; CHEMBL294431; DTXSID6044357; FEMA 2099; PARA METHOXYBENZYL ALCOHOL; NSC2151; 1-methoxy-4-hydroxymethyl-benzene; CHEBI:193647; ZINC388232; BCP26725; STR00774; Tox21_301115; Tox21_302521; BBL027471; STL146342; Anisyl alcohol, >=98%, FCC, FG; AKOS000249369; Anisyl alcohol, natural, >=98%, FG; CS-W016493; PB47798; PS-4037; NCGC00248292-01; NCGC00255015-01; NCGC00256684-01; CAS-105-13-5; CAS-1331-81-3; DB-003500; 4-Methoxybenzyl alcohol, analytical standard; AM20020146; FT-0618922; FT-0671160; M0107; EN300-16200; Anisyl alcohol 1000 microg/mL in Acetonitrile; D77792; Q548873; J-501422; Z54603098; F0001-0102; JandaJel(TM)-Wang, 100-200 mesh, extent of labeling: 1.0 mmol/g loading, 2 % cross-linked
|
|
| CAS | 105-13-5 | |
| PubChem CID | 7738 | |
| ChEMBL ID | CHEMBL294431 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.16 | ALogp: | 1.1 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.673 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.242 | MDCK Permeability: | 0.00001980 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.034 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.015 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.796 | Plasma Protein Binding (PPB): | 55.98% |
| Volume Distribution (VD): | 1.154 | Fu: | 33.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.852 | CYP1A2-substrate: | 0.934 |
| CYP2C19-inhibitor: | 0.547 | CYP2C19-substrate: | 0.749 |
| CYP2C9-inhibitor: | 0.051 | CYP2C9-substrate: | 0.736 |
| CYP2D6-inhibitor: | 0.299 | CYP2D6-substrate: | 0.897 |
| CYP3A4-inhibitor: | 0.048 | CYP3A4-substrate: | 0.512 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.561 | Half-life (T1/2): | 0.832 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.072 | Human Hepatotoxicity (H-HT): | 0.064 |
| Drug-inuced Liver Injury (DILI): | 0.213 | AMES Toxicity: | 0.031 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.534 | Carcinogencity: | 0.495 |
| Eye Corrosion: | 0.056 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.041 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000310 | 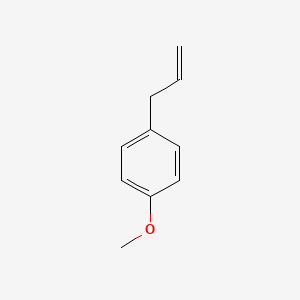 |
0.639 | D05OIS |  |
0.378 | ||
| ENC000318 | 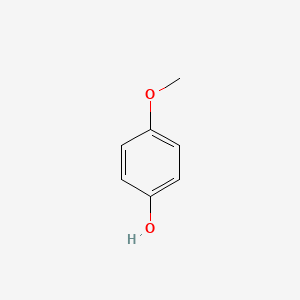 |
0.606 | D02HXS | 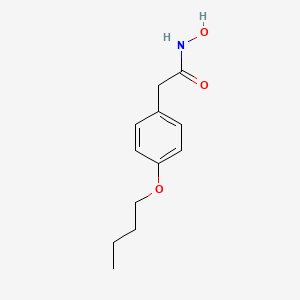 |
0.377 | ||
| ENC000638 | 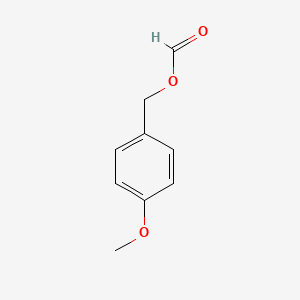 |
0.590 | D0W1RY | 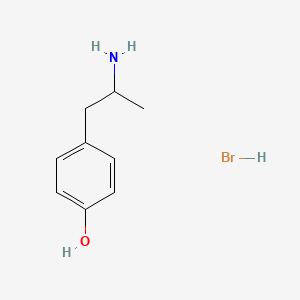 |
0.372 | ||
| ENC000221 | 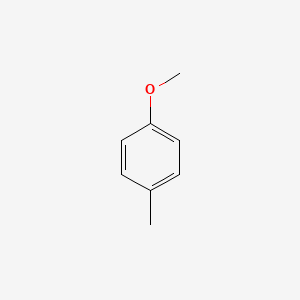 |
0.559 | D05CKR | 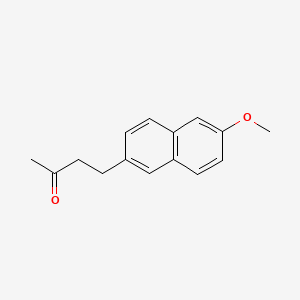 |
0.357 | ||
| ENC002242 | 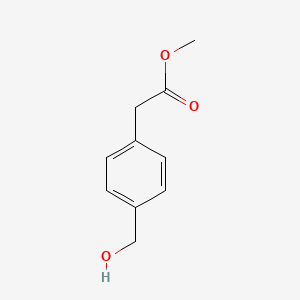 |
0.524 | D02DPU | 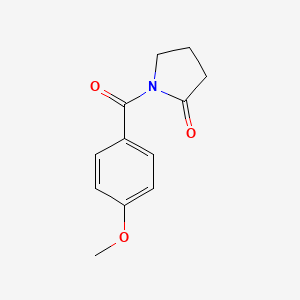 |
0.352 | ||
| ENC005495 | 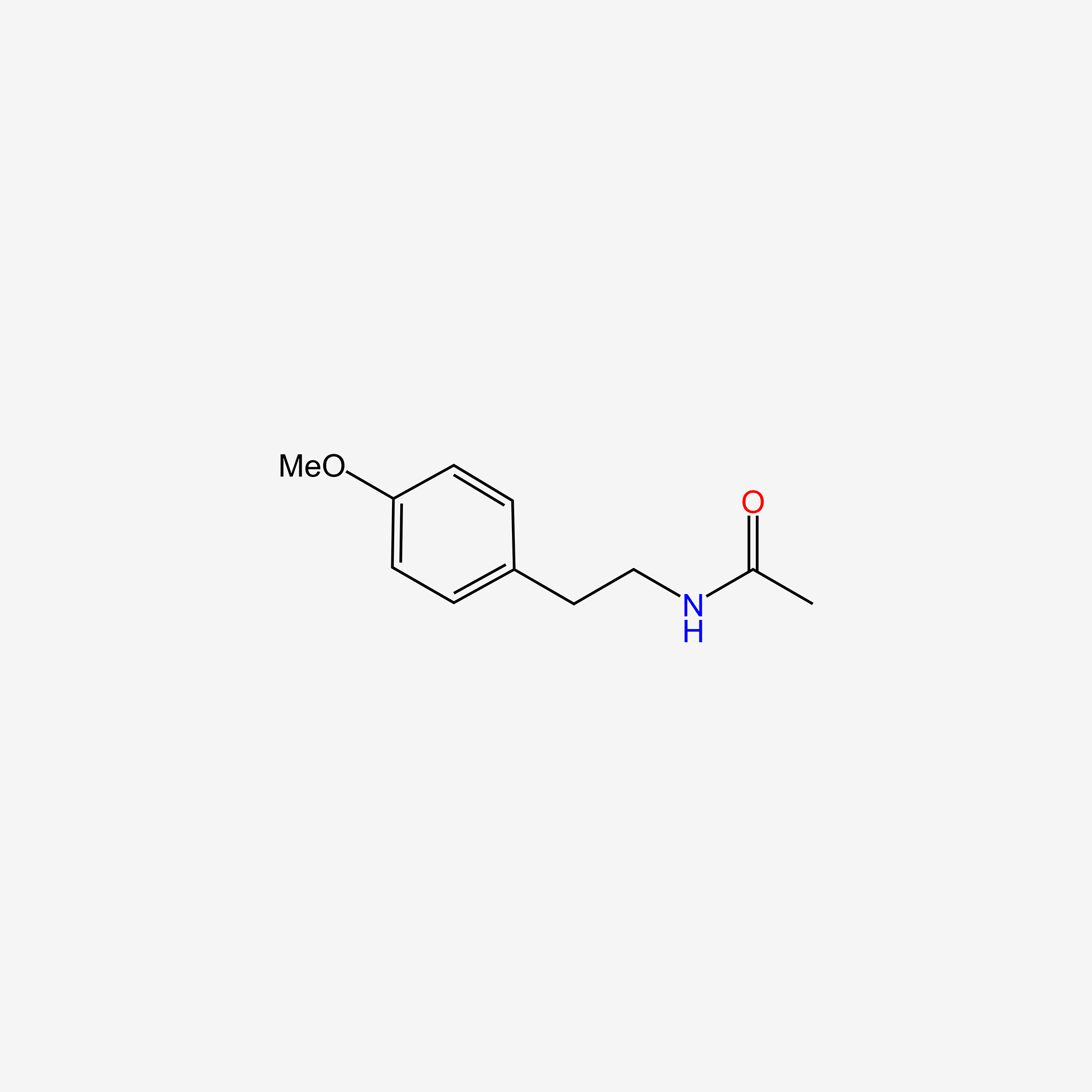 |
0.523 | D0I2MK | 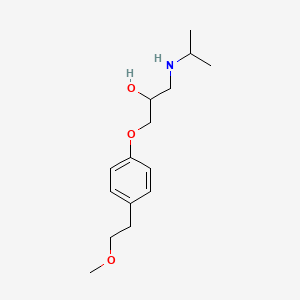 |
0.350 | ||
| ENC001338 | 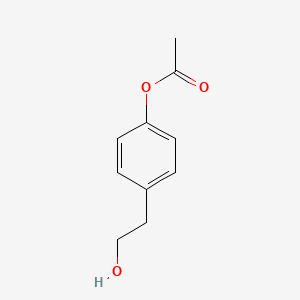 |
0.488 | D08JZS | 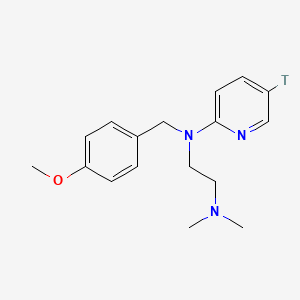 |
0.343 | ||
| ENC000201 | 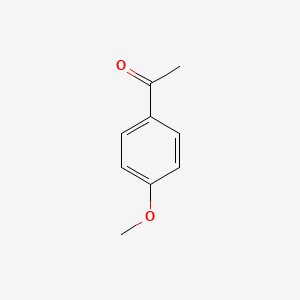 |
0.487 | D0P1UX | 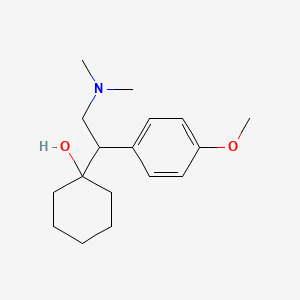 |
0.333 | ||
| ENC001460 | 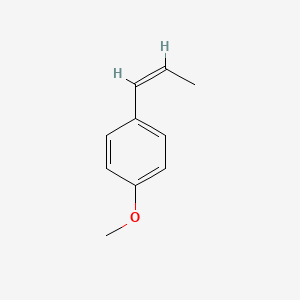 |
0.475 | D01UXC | 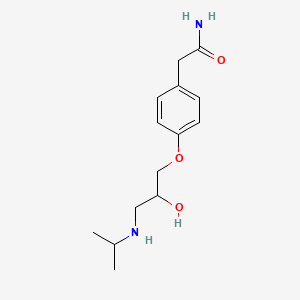 |
0.333 | ||
| ENC000740 | 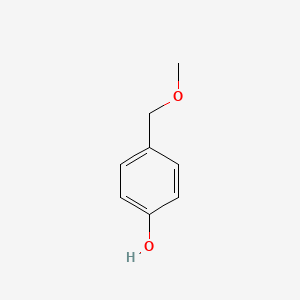 |
0.474 | D0B3QM | 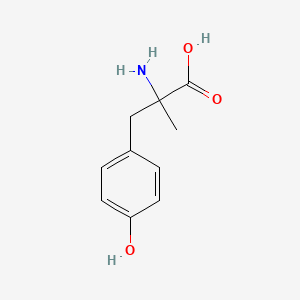 |
0.327 | ||