NPs Basic Information
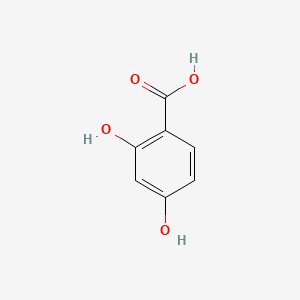
|
Name |
2,4-Dihydroxybenzoic acid
|
| Molecular Formula | C7H6O4 | |
| IUPAC Name* |
2,4-dihydroxybenzoic acid
|
|
| SMILES |
C1=CC(=C(C=C1O)O)C(=O)O
|
|
| InChI |
InChI=1S/C7H6O4/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,8-9H,(H,10,11)
|
|
| InChIKey |
UIAFKZKHHVMJGS-UHFFFAOYSA-N
|
|
| Synonyms |
2,4-dihydroxybenzoic acid; 89-86-1; beta-Resorcylic acid; Benzoic acid, 2,4-dihydroxy-; 4-Carboxyresorcinol; p-Hydroxysalicylic acid; 4-Hydroxysalicylic acid; beta-Resorcinolic acid; 2,4-Dhba; RESORCYLIC ACID, BETA; .beta.-Resorcylic acid; 2,4-Dihydroxy-benzoic acid; 2,4-Resorcylic acid; MFCD00002451; .beta.-Resorcinolic acid; NSC 13564; Coupler 320; LU39SC9JYL; MLS001055408; BRA; NSC-4740; NSC-13564; SMR001227190; 2,4-Dihydroxy benzoic acid; EINECS 201-946-9; UNII-LU39SC9JYL; BRN 1946213; Dihydroxybenzoic acid, 2,4-; AI3-24366; FEMA 3798; RESORCINOL-4-CARBOXYLIC ACID; ss--Resorcylic acid; b-Resorcylic acid, 8CI; Resorcylic acid, .beta.; DSSTox_CID_5074; 2,4-dihyrdoxybenzoic acid; 2.4-dihydroxybenzoic acid; beta resoryclic acid form I; cid_1491; 2,4- dihydroxybenzoic acid; DSSTox_RID_77652; DSSTox_GSID_25074; Oprea1_259729; SCHEMBL28080; 4-10-00-01420 (Beilstein Handbook Reference); 2,4-bis(oxidanyl)benzoic acid; CHEMBL328910; DTXSID0025074; FEMA NO. 3798; BDBM74208; CHEBI:89942; 2,4-hidyroxybenzoic acid form I; NSC4740; 2,4-Dihydroxybenzoic acid, 97%; HMS3039A13; HMS3604D11; ZINC388544; NSC13564; Tox21_202471; .BETA.-RESORCYLIC ACID [MI]; BBL011673; s6293; STK299216; 2,4-Dihydroxybenzoic acid, >=97%; AKOS001434143; CS-W013291; DB02839; HY-W012575; CAS-89-86-1; NCGC00090922-01; NCGC00090922-02; NCGC00260020-01; 2,4-DIHYDROXYBENZOIC ACID [FHFI]; AC-10404; DS-18507; SY014322; D0568; FT-0614326; EN300-06121; D78167; AE-562/41921969; L001160; Q209206; W-100355; F1995-0247; Z104502292; 2,4-Dihydroxybenzoic acid, Vetec(TM) reagent grade, 97%
|
|
| CAS | 89-86-1 | |
| PubChem CID | 1491 | |
| ChEMBL ID | CHEMBL328910 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.12 | ALogp: | 1.6 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 77.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.568 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.459 | MDCK Permeability: | 0.00000531 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.267 |
| 30% Bioavailability (F30%): | 0.986 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.138 | Plasma Protein Binding (PPB): | 51.22% |
| Volume Distribution (VD): | 0.355 | Fu: | 41.71% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.052 | CYP1A2-substrate: | 0.082 |
| CYP2C19-inhibitor: | 0.039 | CYP2C19-substrate: | 0.046 |
| CYP2C9-inhibitor: | 0.172 | CYP2C9-substrate: | 0.194 |
| CYP2D6-inhibitor: | 0.027 | CYP2D6-substrate: | 0.141 |
| CYP3A4-inhibitor: | 0.076 | CYP3A4-substrate: | 0.039 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.11 | Half-life (T1/2): | 0.941 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.041 | Human Hepatotoxicity (H-HT): | 0.548 |
| Drug-inuced Liver Injury (DILI): | 0.864 | AMES Toxicity: | 0.015 |
| Rat Oral Acute Toxicity: | 0.214 | Maximum Recommended Daily Dose: | 0.007 |
| Skin Sensitization: | 0.252 | Carcinogencity: | 0.044 |
| Eye Corrosion: | 0.035 | Eye Irritation: | 0.959 |
| Respiratory Toxicity: | 0.505 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000097 | 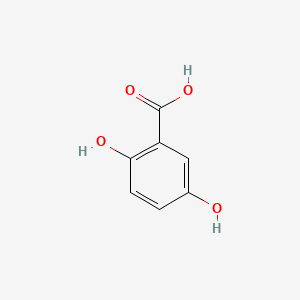 |
0.758 | D01WJL | 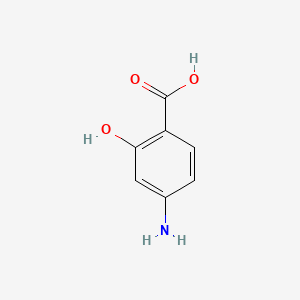 |
0.657 | ||
| ENC000344 | 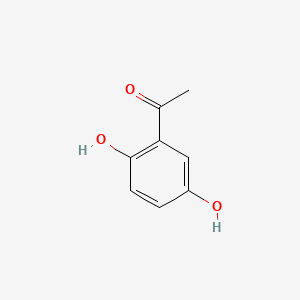 |
0.568 | D0C4YC | 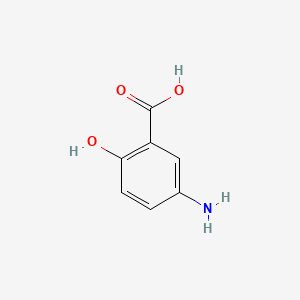 |
0.526 | ||
| ENC000002 | 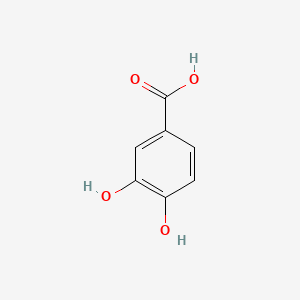 |
0.526 | D07HBX |  |
0.514 | ||
| ENC000103 | 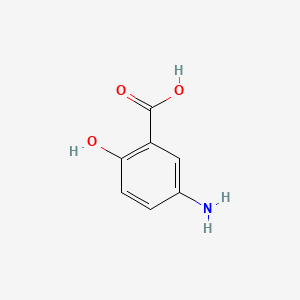 |
0.526 | D0S2BT | 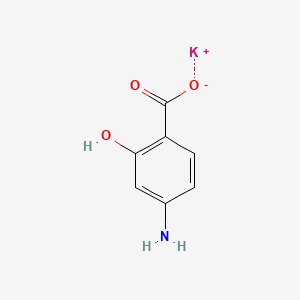 |
0.439 | ||
| ENC004624 | 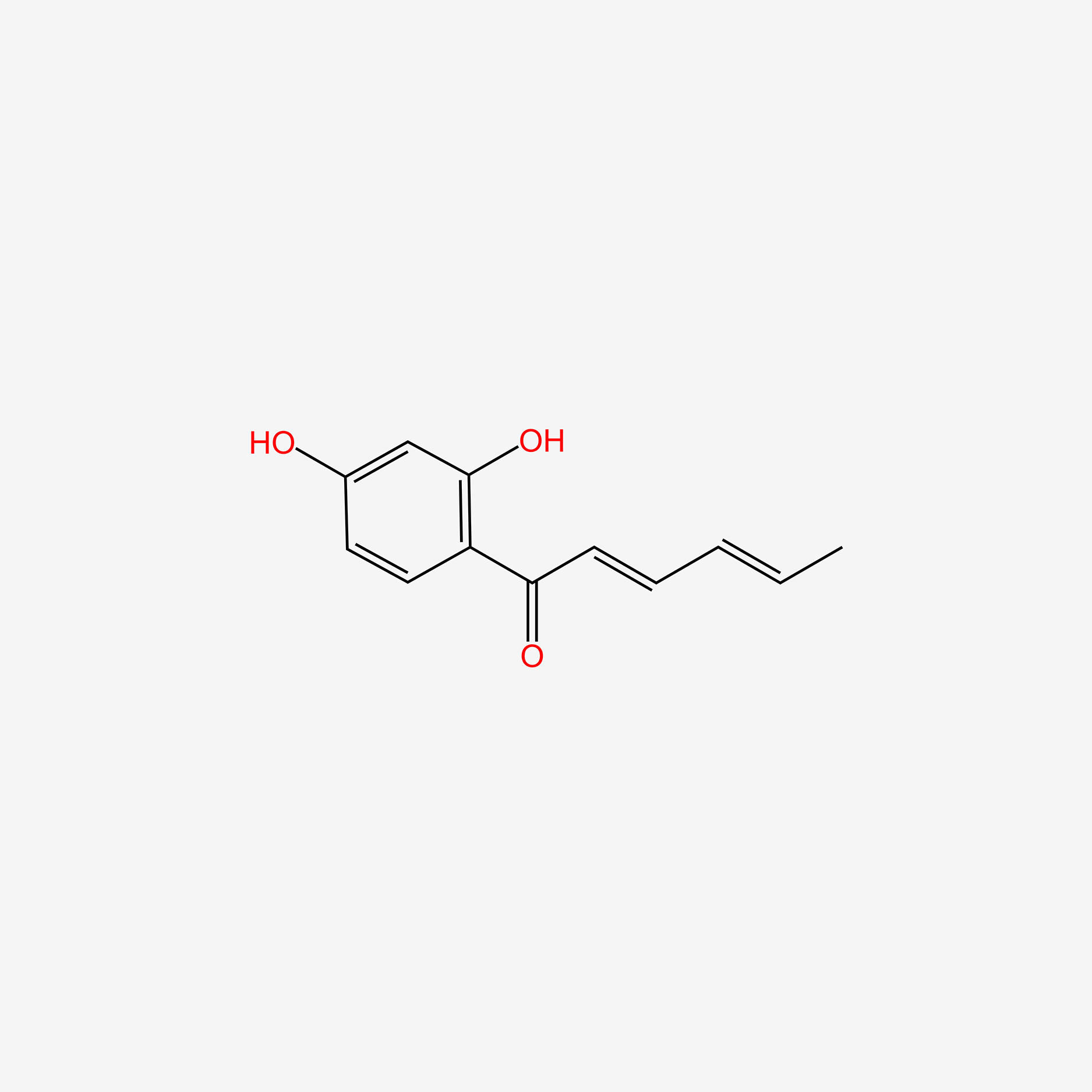 |
0.522 | D0V9EN |  |
0.422 | ||
| ENC004178 | 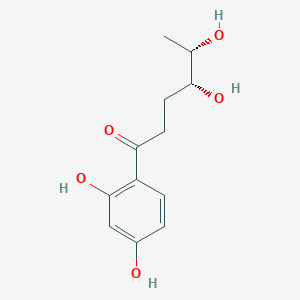 |
0.510 | D08HVR | 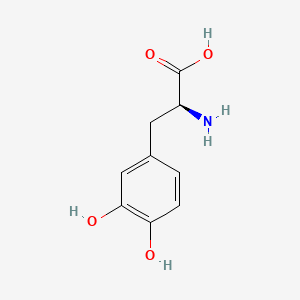 |
0.404 | ||
| ENC002464 | 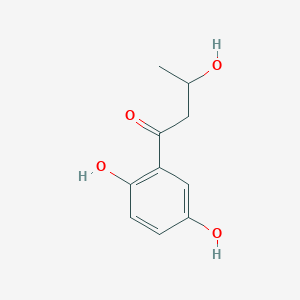 |
0.500 | D0I3RO | 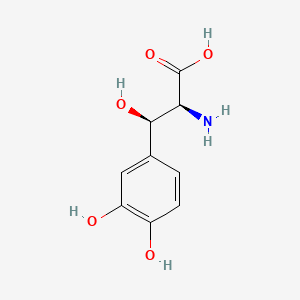 |
0.388 | ||
| ENC002913 | 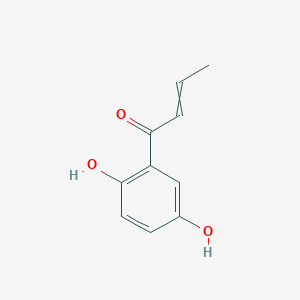 |
0.488 | D0BA6T | 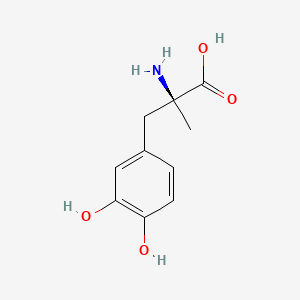 |
0.388 | ||
| ENC000696 | 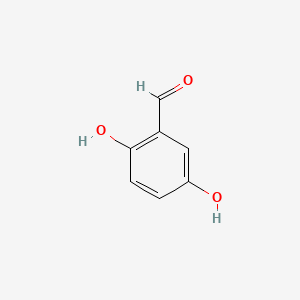 |
0.474 | D0P7JZ | 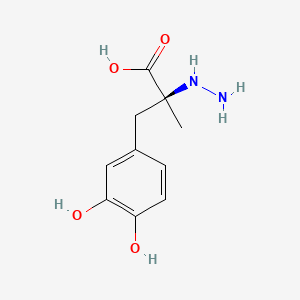 |
0.365 | ||
| ENC000985 | 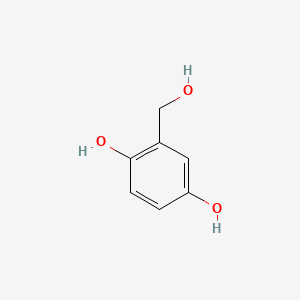 |
0.474 | D0F5ZM | 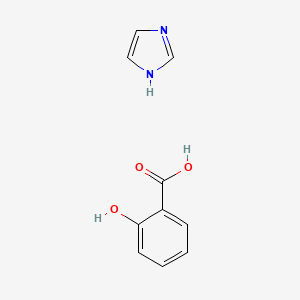 |
0.365 | ||