NPs Basic Information
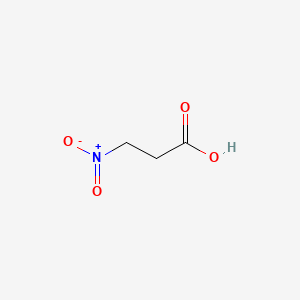
|
Name |
3-Nitropropionic acid
|
| Molecular Formula | C3H5NO4 | |
| IUPAC Name* |
3-nitropropanoic acid
|
|
| SMILES |
C(C[N+](=O)[O-])C(=O)O
|
|
| InChI |
InChI=1S/C3H5NO4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)
|
|
| InChIKey |
WBLZUCOIBUDNBV-UHFFFAOYSA-N
|
|
| Synonyms |
3-nitropropionic acid; 3-Nitropropanoic acid; 504-88-1; Bovinocidin; Hiptagenic acid; beta-Nitropropionic acid; 3-Nitropropionate; Propanoic acid, 3-nitro-; Propionic acid, 3-nitro-; 3-NP acid; beta-Nitropropanoic acid; NCI-C03076; NSC 64266; 3-nitro-propionic acid; BOVINOCIDIN (3-nitropropionic acid); NITROPROPIONIC ACID, BETA; .beta.-Nitropropionic acid; 3-NPA; beta-Nitropropanoate; 3-Nitroporpionic Acid; CHEBI:16348; QY4L0FOX0D; BNP; CHEMBL451226; NSC64266; NSC-64266; DSSTox_CID_982; DSSTox_RID_75904; 3-nitro-1-propionate; DSSTox_GSID_20982; CAS-504-88-1; CCRIS 454; HSDB 4147; Nitropropionic aci, beta; SR-01000076030; EINECS 208-003-0; UNII-QY4L0FOX0D; BRN 1759889; MFCD00007406; b-nitropropionic acid; Spectrum_001674; 3-Nitro-Propanoic acid; SpecPlus_000692; beta -nitropropionic acid; Nitropropionic acid, 3-; Spectrum2_000876; Spectrum3_000993; Spectrum4_001119; Spectrum5_001895; Lopac-N-5636; WLN: WN2VQ; Nitropropionic acid, .beta.; 3-Nitropropionic acid, 8CI; Lopac0_000838; SCHEMBL70072; BSPBio_002685; KBioGR_001598; KBioSS_002154; 4-02-00-00771 (Beilstein Handbook Reference); MLS001066410; 3-Nitropropionic acid, 97%; DivK1c_006788; SPECTRUM1504206; SPBio_000951; 3-NP; DTXSID1020982; 3-Nitropropionic acid, >=97%; BDBM82201; KBio1_001732; KBio2_002154; KBio2_004722; KBio2_007290; KBio3_001905; HMS2235N03; HMS3262H17; HMS3369D16; HMS3865D03; ZINC895862; Propanoic acid, 3-nitro- (9CI); BCP15066; Tox21_202460; Tox21_303007; Tox21_500838; BDBM50480795; CCG-40325; s3652; 3-NITROPROPIONIC ACID [HSDB]; AKOS006221915; AKOS015833440; 504-88-1 (FREE ACID); CS-W013591; HY-W012875; LP00838; SDCCGMLS-0066769.P001; SDCCGSBI-0050815.P003; 3-Nitropropanoic acid; 3-nitropropanoate; NCGC00015744-01; NCGC00015744-02; NCGC00015744-03; NCGC00015744-04; NCGC00015744-05; NCGC00015744-06; NCGC00015744-07; NCGC00015744-11; NCGC00094169-01; NCGC00094169-02; NCGC00094169-03; NCGC00094169-04; NCGC00256389-01; NCGC00260009-01; NCGC00261523-01; SMR000471863; 3-Nitropropionic acid - CAS 504-88-1; DB-008848; EU-0100838; FT-0632243; C05669; F17743; N 5636; 2-(4-Nitrophenylamino)thiazole-4-carboxylicacid; Q223104; SR-01000076030-1; SR-01000076030-5
|
|
| CAS | 504-88-1 | |
| PubChem CID | 1678 | |
| ChEMBL ID | CHEMBL451226 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 119.08 | ALogp: | -0.5 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.424 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.44 | MDCK Permeability: | 0.00177134 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.015 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.215 | Plasma Protein Binding (PPB): | 34.72% |
| Volume Distribution (VD): | 0.273 | Fu: | 68.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.01 | CYP1A2-substrate: | 0.069 |
| CYP2C19-inhibitor: | 0.042 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.851 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.166 |
| CYP3A4-inhibitor: | 0.011 | CYP3A4-substrate: | 0.024 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.987 | Half-life (T1/2): | 0.857 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.843 |
| Drug-inuced Liver Injury (DILI): | 0.072 | AMES Toxicity: | 0.801 |
| Rat Oral Acute Toxicity: | 0.227 | Maximum Recommended Daily Dose: | 0.149 |
| Skin Sensitization: | 0.526 | Carcinogencity: | 0.099 |
| Eye Corrosion: | 0.975 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.083 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000677 |  |
0.400 | D06VNK |  |
0.379 | ||
| ENC000018 |  |
0.400 | D0EP8X | 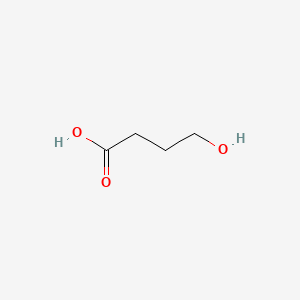 |
0.357 | ||
| ENC001187 | 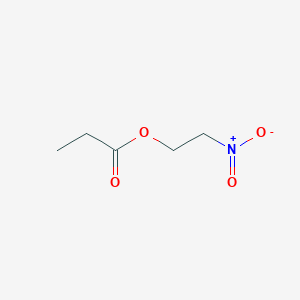 |
0.394 | D0Y7ZD | 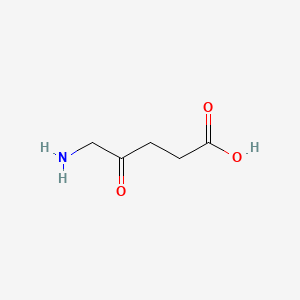 |
0.344 | ||
| ENC000062 | 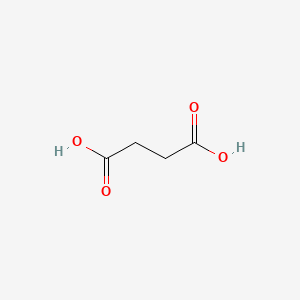 |
0.379 | D0O4GY | 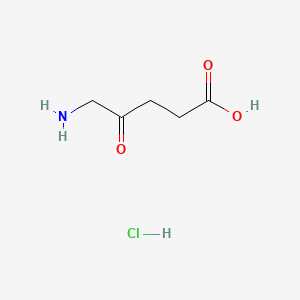 |
0.333 | ||
| ENC000639 | 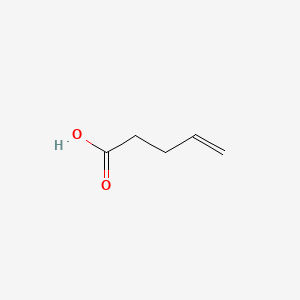 |
0.357 | D00ENY | 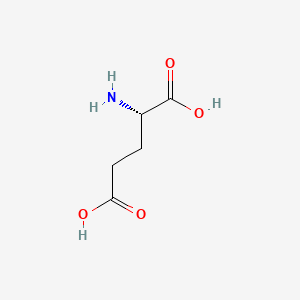 |
0.324 | ||
| ENC000735 | 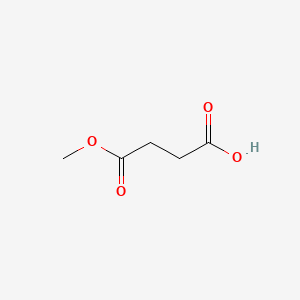 |
0.344 | D0R3QY |  |
0.303 | ||
| ENC000445 | 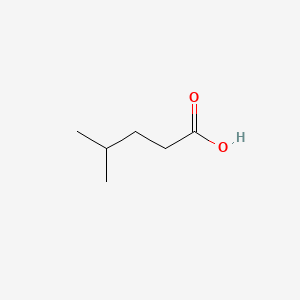 |
0.333 | D0FD0H | 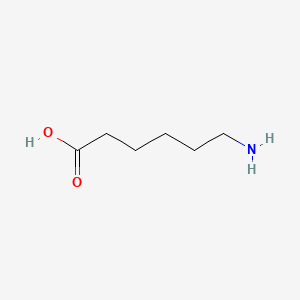 |
0.294 | ||
| ENC000315 | 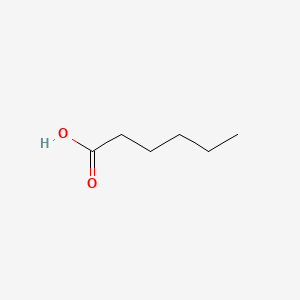 |
0.323 | D0M8AB | 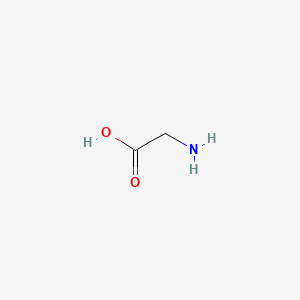 |
0.292 | ||
| ENC000643 |  |
0.323 | D0Z0MG |  |
0.262 | ||
| ENC000795 |  |
0.297 | D02FLB |  |
0.261 | ||