NPs Basic Information

|
Name |
L-2-Aminoadipic acid
|
| Molecular Formula | C6H11NO4 | |
| IUPAC Name* |
(2S)-2-aminohexanedioic acid
|
|
| SMILES |
C(C[C@@H](C(=O)O)N)CC(=O)O
|
|
| InChI |
InChI=1S/C6H11NO4/c7-4(6(10)11)2-1-3-5(8)9/h4H,1-3,7H2,(H,8,9)(H,10,11)/t4-/m0/s1
|
|
| InChIKey |
OYIFNHCXNCRBQI-BYPYZUCNSA-N
|
|
| Synonyms |
L-2-Aminoadipic acid; 1118-90-7; (S)-2-aminohexanedioic acid; (2S)-2-aminohexanedioic acid; L-alpha-Aminoadipic acid; L-alpha-Aminoadipate; L-2-Aminoadipate; (S)-2-Aminoadipic acid; L-2-Aminohexanedioate; hexanedioic acid, 2-amino-, (2S)-; l-2-amino adipic acid; homoglutamic acid; 2-Aminoadipic acid, L-; h-l-2-aad-oh; Hexanedioic acid, 2-amino-, (S)-; Aminoadipate; CHEMBL88804; 88ZH74L7SR; CHEBI:37023; L-aminoadipic acid; L-Homoglutamic acid; MFCD00002636; DL-alpha-Aminoadipate; Adipic acid, 2-amino-; l-a-aminoadipic acid; L-.alpha.-Aminoadipic acid; alpha-Aminoadipate, L-; L-alpha-Aminoadipatic acid; UNII-88ZH74L7SR; a-Aminoadipate; a-Aminoadipic acid; DL-a-Aminoadipate; UN1; DL-2-Aminoadipate; h-2-aad-oh; DL-a-Aminoadipic acid; l-a-aminohexanoic acid; (S)-Aminoadipic Acid; DL-2-Aminohexanedioate; alpha-Amino-adipic acid; L-2-amino-adipic acid; ALHA-AMINOADIPATE; (S)-2-Aminoadipicacid; aminoadipic acid, l-2-; L -alpha-aminoadipic acid; (+/-)-2-Aminoadipate; L-2-Aminohexanedioic acid; (2S)-2-aminoadipic acid; L- alpha -Aminoadipic acid; l-alpha-aminohexanedioic acid; Lopac0_000091; SCHEMBL97323; (S)-2-amino-hexanedioic acid; L-.ALPHA.-AMINOADIPATE; (2S)-2-Amino-hexanedioic Acid; DTXSID801017096; HMS3260C04; ZINC1532798; Tox21_500091; BDBM50052553; AKOS015920301; CCG-204186; CS-W014381; LP00091; SDCCGSBI-0050079.P002; HEXANEDIOIC ACID, 2-AMINO-, L-; L-2-Aminoadipic acid, >=98% (TLC); NCGC00093593-01; NCGC00093593-02; NCGC00093593-03; NCGC00093593-04; NCGC00260776-01; AS-13597; EU-0100091; EN300-97272; A 7275; C00956; 118A907; SR-01000075684; J-002657; SR-01000075684-1; Q27104392; Aad; (S)-2-Aminohexanedioic acid; L-Homoglutamic acid; 9EF7020B-8995-4D0E-BE44-34D8F18766DF
|
|
| CAS | 1118-90-7 | |
| PubChem CID | 92136 | |
| ChEMBL ID | CHEMBL88804 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 161.16 | ALogp: | -3.1 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 101.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.53 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.025 | MDCK Permeability: | 0.00566659 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.05 |
| Human Intestinal Absorption (HIA): | 0.09 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.483 | Plasma Protein Binding (PPB): | 12.35% |
| Volume Distribution (VD): | 0.326 | Fu: | 78.91% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.005 | CYP1A2-substrate: | 0.023 |
| CYP2C19-inhibitor: | 0.036 | CYP2C19-substrate: | 0.042 |
| CYP2C9-inhibitor: | 0.017 | CYP2C9-substrate: | 0.479 |
| CYP2D6-inhibitor: | 0.039 | CYP2D6-substrate: | 0.146 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.005 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.53 | Half-life (T1/2): | 0.695 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.053 |
| Drug-inuced Liver Injury (DILI): | 0.013 | AMES Toxicity: | 0.025 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.009 |
| Skin Sensitization: | 0.134 | Carcinogencity: | 0.059 |
| Eye Corrosion: | 0.006 | Eye Irritation: | 0.111 |
| Respiratory Toxicity: | 0.081 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000550 | 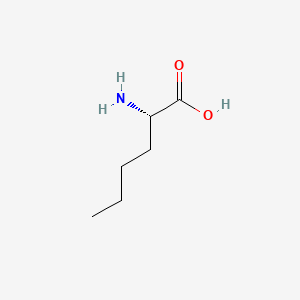 |
0.545 | D00ENY | 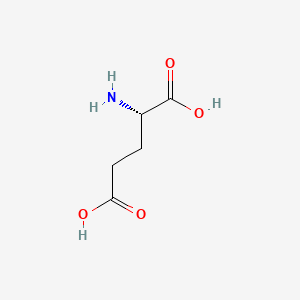 |
0.767 | ||
| ENC000137 | 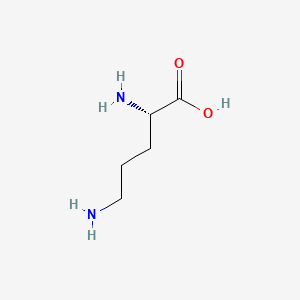 |
0.545 | D01JIA | 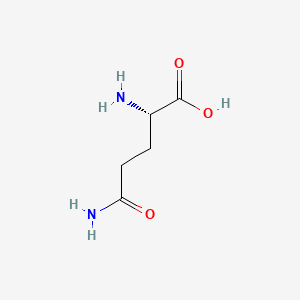 |
0.559 | ||
| ENC000123 | 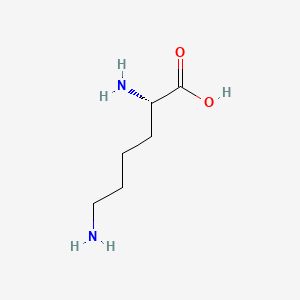 |
0.500 | D0X5SI | 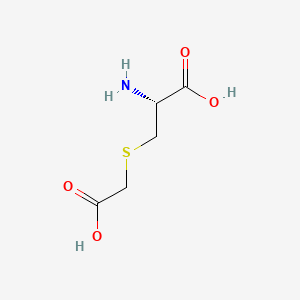 |
0.514 | ||
| ENC000937 | 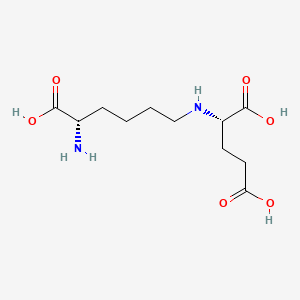 |
0.500 | D0F5DO | 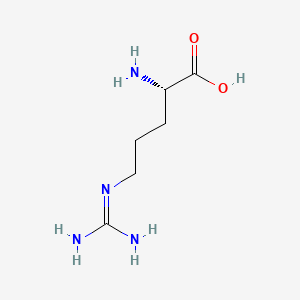 |
0.475 | ||
| ENC000142 | 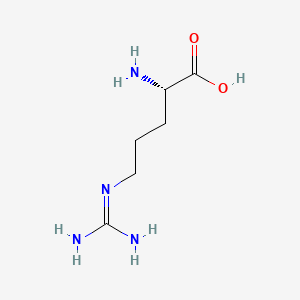 |
0.475 | D06VNK | 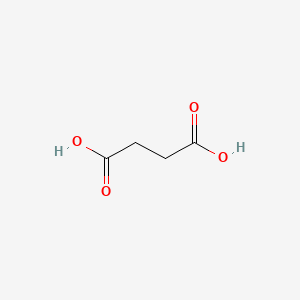 |
0.455 | ||
| ENC000062 | 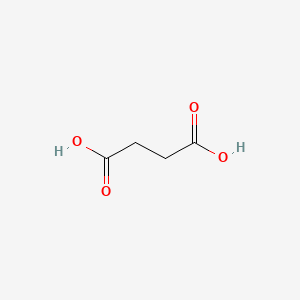 |
0.455 | D0Z0MG | 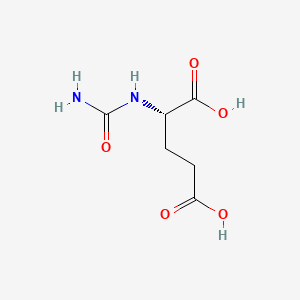 |
0.452 | ||
| ENC000760 | 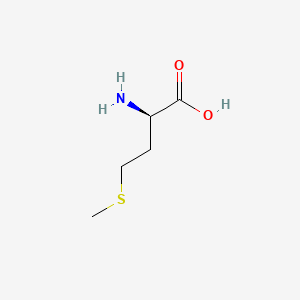 |
0.417 | D0EP8X | 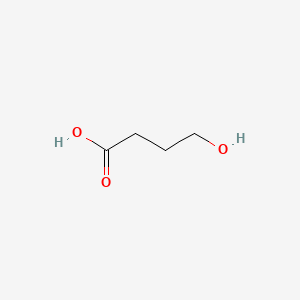 |
0.438 | ||
| ENC000075 |  |
0.400 | D0Y7ZD | 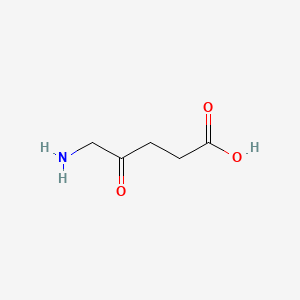 |
0.417 | ||
| ENC000315 | 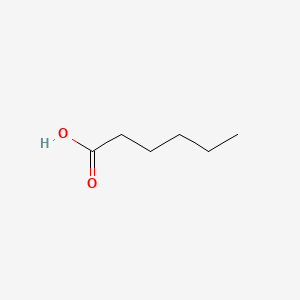 |
0.361 | D01OPV | 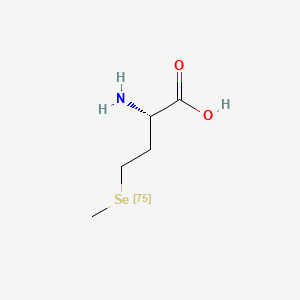 |
0.417 | ||
| ENC005079 | 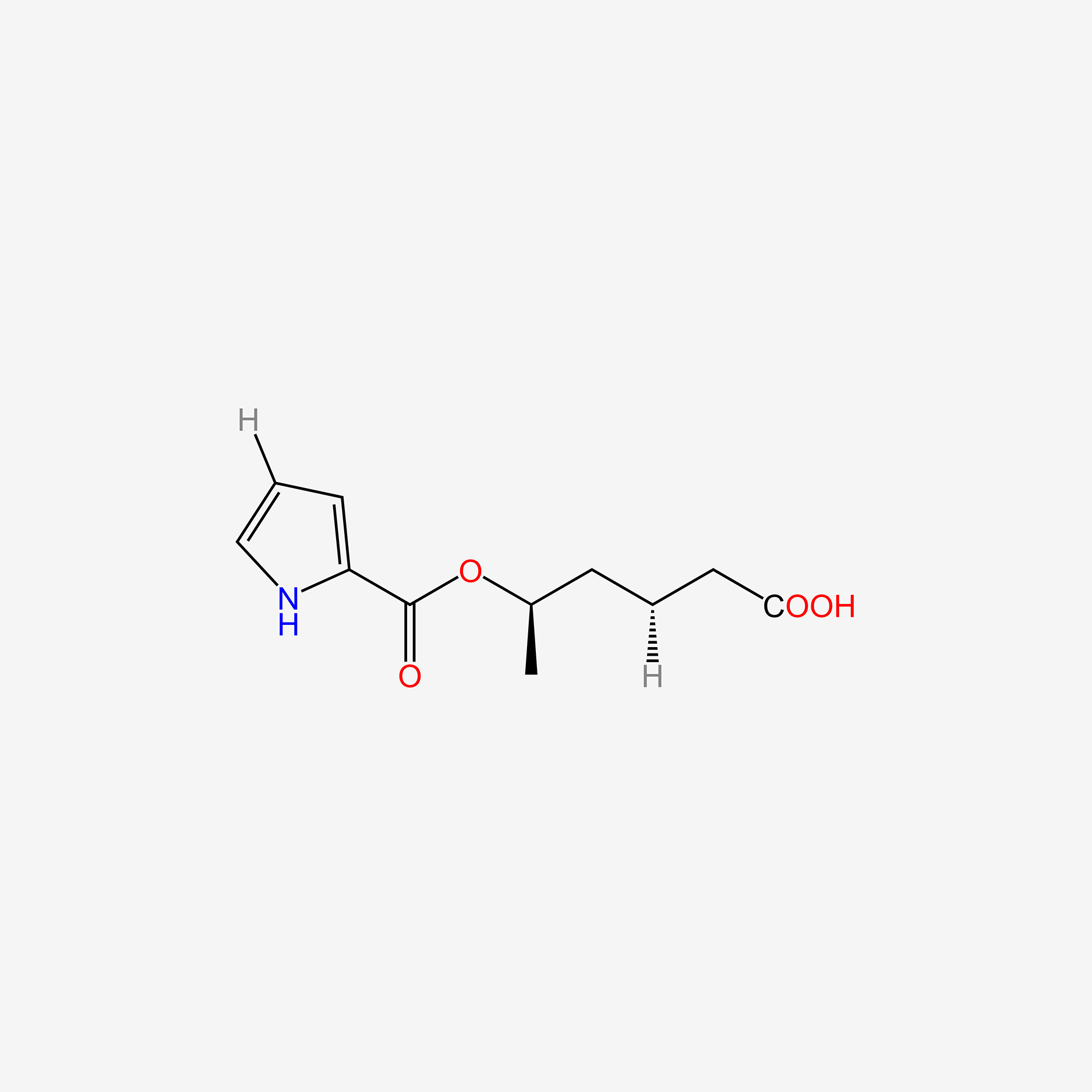 |
0.358 | D02UDJ | 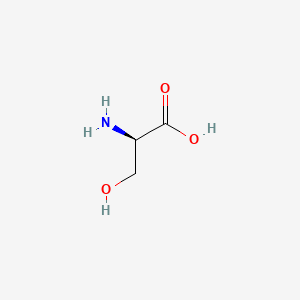 |
0.406 | ||