NPs Basic Information
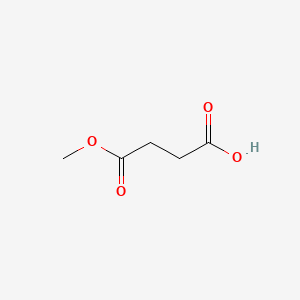
|
Name |
Monomethyl succinate
|
| Molecular Formula | C5H8O4 | |
| IUPAC Name* |
4-methoxy-4-oxobutanoic acid
|
|
| SMILES |
COC(=O)CCC(=O)O
|
|
| InChI |
InChI=1S/C5H8O4/c1-9-5(8)3-2-4(6)7/h2-3H2,1H3,(H,6,7)
|
|
| InChIKey |
JDRMYOQETPMYQX-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methoxy-4-oxobutanoic acid; Monomethyl succinate; 3878-55-5; Mono-Methyl Succinate; Succinic acid monomethyl ester; Methyl hydrogen succinate; Succinic acid, monomethyl ester; 3-Carbomethoxypropanoic acid; Butanedioic acid, monomethyl ester; mono-Methyl hydrogen succinate; MFCD00002788; YA2V724S0A; butanedioic acid 1-methyl ester; butanedioic acid monomethyl ester; Butanedioic acid, 1-methyl ester; NSC-511; UNII-YA2V724S0A; 3-(methoxycarbonyl)propanoic acid; NSC 511; EINECS 223-408-2; Succinic acid 1-methyl; AI3-03389; DSSTox_CID_24425; DSSTox_RID_80218; Monomethyl hydrogen succinate; DSSTox_GSID_44425; succinic acid 4-methyl ester; SCHEMBL164072; 3-methoxycarbonylpropionic acid; NSC511; DTXSID7044425; 4-Methoxy-4-oxobutanoic acid #; BDBM82193; CHEBI:75146; JDRMYOQETPMYQX-UHFFFAOYSA-; 4-(methyloxy)-4-oxobutanoic acid; ZINC152993; Tox21_302188; mono-Methyl hydrogen succinate, 95%; STL068961; ACIDYELLOW25(C.I.18835); AKOS000264979; CS-W011096; GS-3418; NCGC00257549-01; BP-23493; SY009523; CAS-3878-55-5; AM20100028; BB 0254379; FT-0618900; M3262; EN300-24949; A855318; J-515649; Q21045268; Z57663583; F1107-0326
|
|
| CAS | 3878-55-5 | |
| PubChem CID | 77487 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 132.11 | ALogp: | 0.0 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 63.6 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.565 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.401 | MDCK Permeability: | 0.00222148 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.023 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.93 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.966 | Plasma Protein Binding (PPB): | 14.47% |
| Volume Distribution (VD): | 0.24 | Fu: | 78.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.011 | CYP1A2-substrate: | 0.134 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.058 |
| CYP2C9-inhibitor: | 0.003 | CYP2C9-substrate: | 0.878 |
| CYP2D6-inhibitor: | 0.017 | CYP2D6-substrate: | 0.192 |
| CYP3A4-inhibitor: | 0.012 | CYP3A4-substrate: | 0.045 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.75 | Half-life (T1/2): | 0.858 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.006 | Human Hepatotoxicity (H-HT): | 0.117 |
| Drug-inuced Liver Injury (DILI): | 0.167 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.006 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.131 | Carcinogencity: | 0.061 |
| Eye Corrosion: | 0.984 | Eye Irritation: | 0.965 |
| Respiratory Toxicity: | 0.029 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000234 | 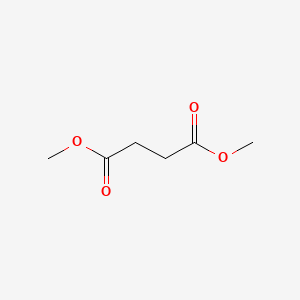 |
0.581 | D0OL6O | 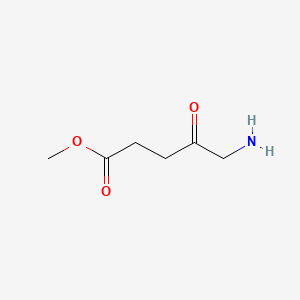 |
0.581 | ||
| ENC000062 |  |
0.536 | D06VNK | 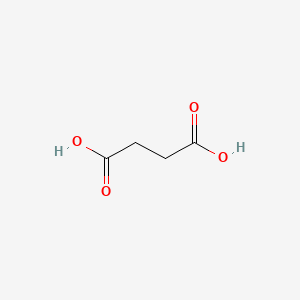 |
0.536 | ||
| ENC001036 | 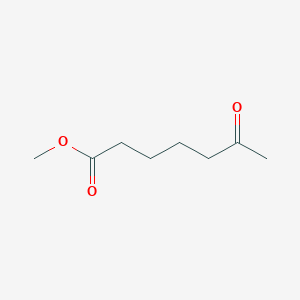 |
0.486 | D0Y7ZD | 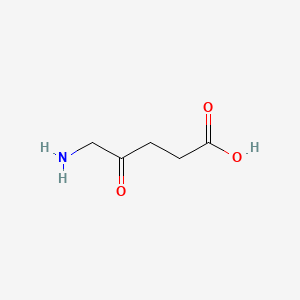 |
0.484 | ||
| ENC003372 | 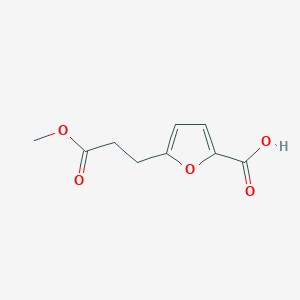 |
0.419 | D0O4GY | 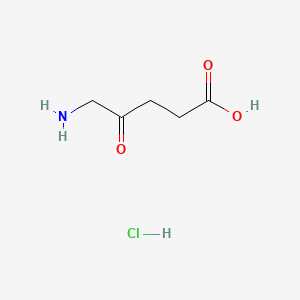 |
0.469 | ||
| ENC000018 | 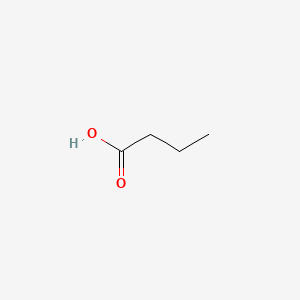 |
0.407 | D00ENY | 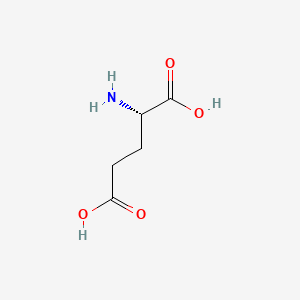 |
0.371 | ||
| ENC001253 | 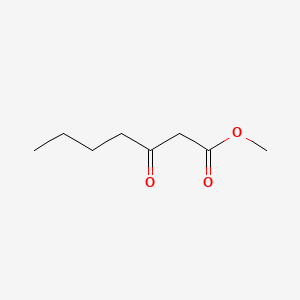 |
0.405 | D0G4JI | 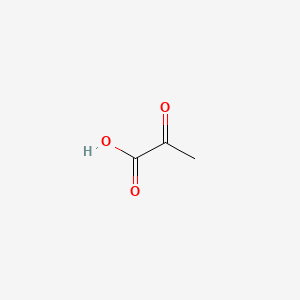 |
0.333 | ||
| ENC000516 | 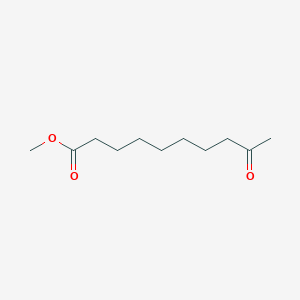 |
0.386 | D0EP8X | 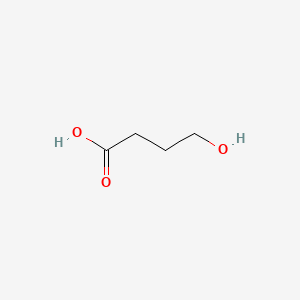 |
0.323 | ||
| ENC000235 |  |
0.382 | D0E4WR | 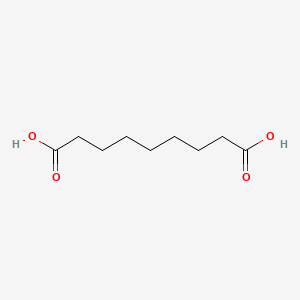 |
0.318 | ||
| ENC006075 | 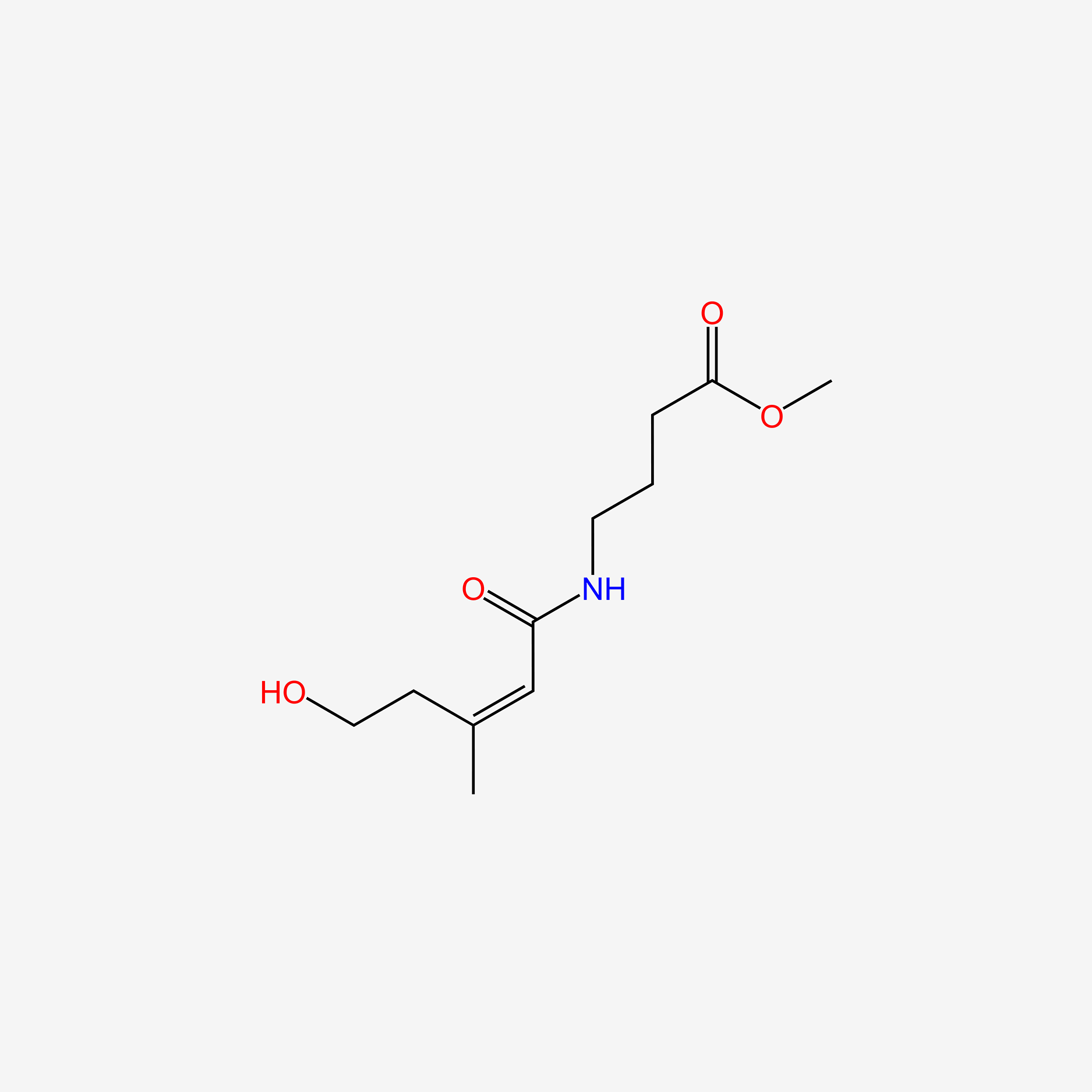 |
0.375 | D0Z0MG | 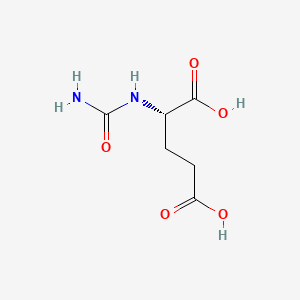 |
0.302 | ||
| ENC000643 | 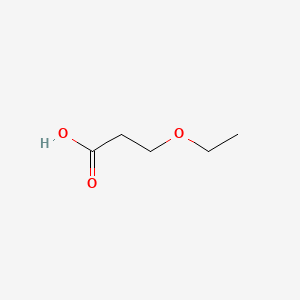 |
0.375 | D07SJT | 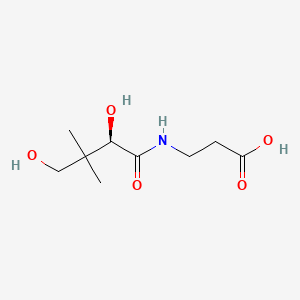 |
0.298 | ||