NPs Basic Information
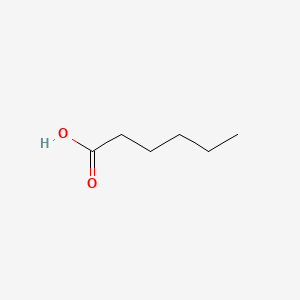
|
Name |
Hexanoic acid
|
| Molecular Formula | C6H12O2 | |
| IUPAC Name* |
hexanoic acid
|
|
| SMILES |
CCCCCC(=O)O
|
|
| InChI |
InChI=1S/C6H12O2/c1-2-3-4-5-6(7)8/h2-5H2,1H3,(H,7,8)
|
|
| InChIKey |
FUZZWVXGSFPDMH-UHFFFAOYSA-N
|
|
| Synonyms |
HEXANOIC ACID; Caproic acid; 142-62-1; n-Hexanoic acid; n-Caproic acid; Capronic acid; Butylacetic acid; Pentylformic acid; Hexoic acid; 1-Hexanoic acid; n-Hexylic acid; n-Hexoic acid; Pentiformic acid; 1-Pentanecarboxylic acid; Pentanecarboxylic acid; Hexacid 698; Hexylic acid; Kyselina kapronova; FEMA No. 2559; NSC 8266; NCIOpen2_005355; C6:0; CHEMBL14184; CH3-[CH2]4-COOH; 1F8SN134MX; CHEBI:30776; NSC8266; butylacetate; capronate; hexylate; pentylformate; NSC-8266; n-caproate; n-hexoate; n-hexylate; HEXANOIC ACID (CAPROIC ACID); 1-hexanoate; 1-pentanecarboxylate; DSSTox_CID_1607; Hexanoic acid (natural); DSSTox_RID_76233; DSSTox_GSID_21607; 68603-84-9; 70248-25-8; Kyselina kapronova [Czech]; CAS-142-62-1; Acid C6; CCRIS 1347; NSC-35598; HSDB 6813; EINECS 205-550-7; MFCD00004421; UN2829; BRN 0773837; UNII-1F8SN134MX; AI3-07701; hexansäure; Nat.Hexanoic Acid; 6NA; EINECS 274-509-3; Caproic Acid,(S); 58454-02-7; ethyl 4-butanoic acid; Hexanoic acid, 99%; methyl 5-pentanoic acid; Pentane-1-carboxylic acid; Hexanoic acid, >=99%; n-C5H11COOH; Hexanoic acid Caproic acid; bmse000351; EC 205-550-7; SCHEMBL3867; WLN: QV5; CH3(CH2)4COOH; CAPROIC ACID [HSDB]; CAPROIC ACID [INCI]; HEXANOIC ACID [FCC]; HEXANOIC ACID [FHFI]; N-CAPROIC ACID [MI]; 4-02-00-00917 (Beilstein Handbook Reference); DTXSID7021607; Hexanoic acid-1,2-[13C2]; BDBM16433; 1-PENTANE CARBOXYLIC ACID; Hexanoic acid, analytical standard; STR10048; ZINC1529230; EINECS 267-013-3; EINECS 271-676-4; Tox21_201517; Tox21_300406; LMFA01010006; Hexanoic acid, >=98%, FCC, FG; AKOS000119844; FA(6:0); Caproic acid [UN2829] [Corrosive]; Hexanoic acid, natural, >=98%, FCC; NCGC00248020-01; NCGC00248020-02; NCGC00254504-01; NCGC00259067-01; Hexanoic acid, purum, >=98.0% (GC); Hexanoic acid 10 microg/mL in Acetonitrile; FT-0659402; FT-0777869; H0105; Hexanoic acid, natural, >=98%, FCC, FG; EN300-21589; C01585; EC 271-676-4; Q422597; J-007673; 25401AB4-1ECB-481F-AC91-EAAFC9329BDD; CAPROIC ACID (CONSTITUENT OF SAW PALMETTO) [DSC]; Z104503532
|
|
| CAS | 142-62-1 | |
| PubChem CID | 8892 | |
| ChEMBL ID | CHEMBL14184 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 116.16 | ALogp: | 1.9 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.572 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.721 | MDCK Permeability: | 0.00003430 |
| Pgp-inhibitor: | 0.008 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.166 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.878 | Plasma Protein Binding (PPB): | 71.14% |
| Volume Distribution (VD): | 0.245 | Fu: | 37.21% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.042 | CYP1A2-substrate: | 0.425 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.408 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.948 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.221 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.046 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.645 | Half-life (T1/2): | 0.798 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.063 |
| Drug-inuced Liver Injury (DILI): | 0.049 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.13 | Maximum Recommended Daily Dose: | 0.018 |
| Skin Sensitization: | 0.208 | Carcinogencity: | 0.213 |
| Eye Corrosion: | 0.983 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.11 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000030 | 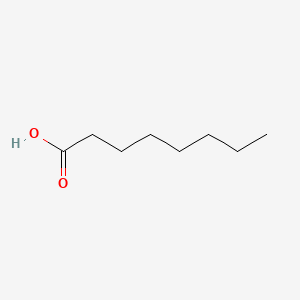 |
0.778 | D0FD0H | 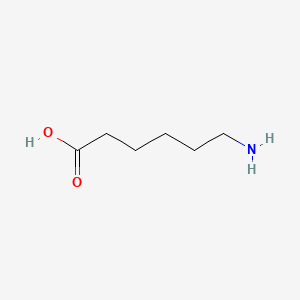 |
0.552 | ||
| ENC000263 | 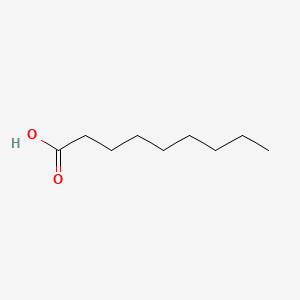 |
0.700 | D0EP8X | 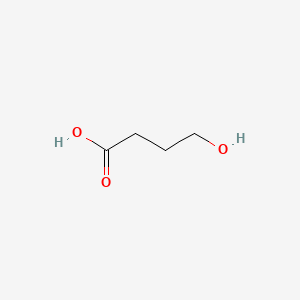 |
0.500 | ||
| ENC000088 | 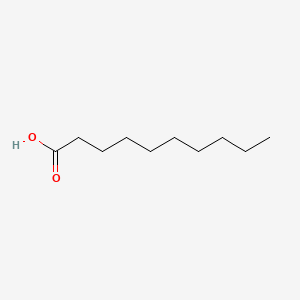 |
0.636 | D0E4WR | 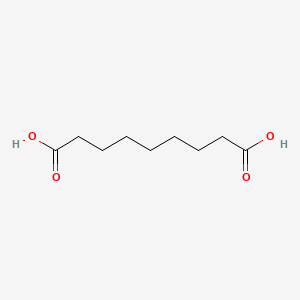 |
0.400 | ||
| ENC000250 | 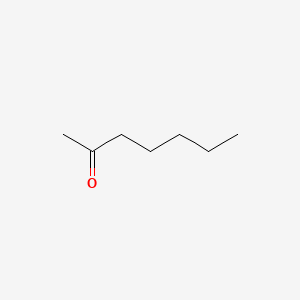 |
0.615 | D0Z5BC |  |
0.390 | ||
| ENC000270 | 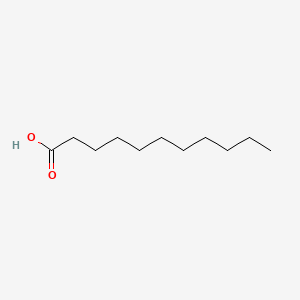 |
0.583 | D01QLH | 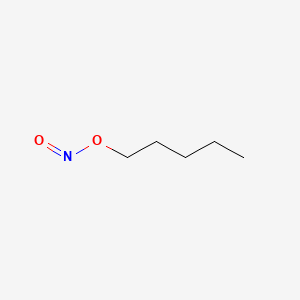 |
0.387 | ||
| ENC000018 | 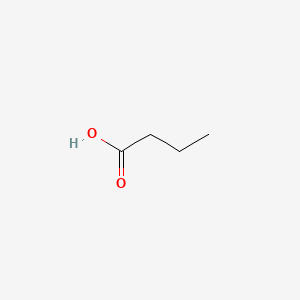 |
0.565 | D0UE9X | 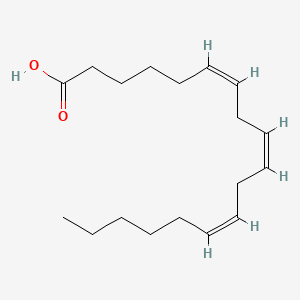 |
0.368 | ||
| ENC001025 | 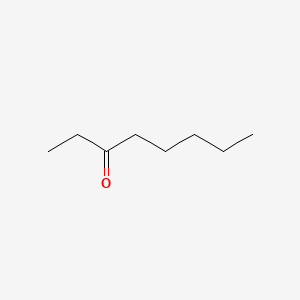 |
0.552 | D0XN8C |  |
0.356 | ||
| ENC000235 |  |
0.552 | D0Y3KG | 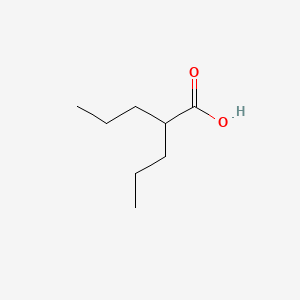 |
0.343 | ||
| ENC000254 | 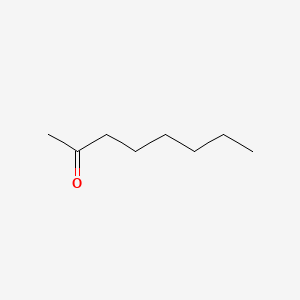 |
0.552 | D0Y7ZD | 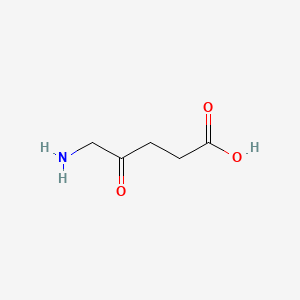 |
0.333 | ||
| ENC000102 | 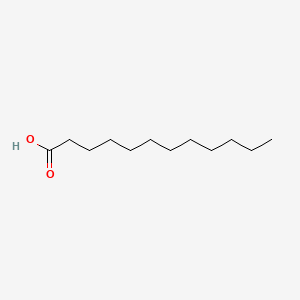 |
0.538 | D0O1TC | 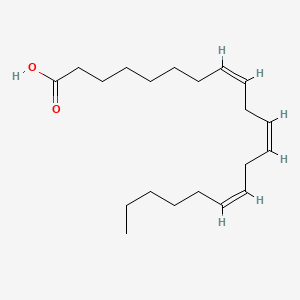 |
0.333 | ||