NPs Basic Information
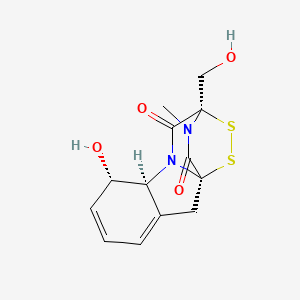
|
Name |
Gliotoxin
|
| Molecular Formula | C13H14N2O4S2 | |
| IUPAC Name* |
(1R,7S,8S,11R)-7-hydroxy-11-(hydroxymethyl)-15-methyl-12,13-dithia-9,15-diazatetracyclo[9.2.2.01,9.03,8]pentadeca-3,5-diene-10,14-dione
|
|
| SMILES |
CN1C(=O)[C@]23CC4=CC=C[C@@H]([C@H]4N2C(=O)[C@]1(SS3)CO)O
|
|
| InChI |
InChI=1S/C13H14N2O4S2/c1-14-10(18)12-5-7-3-2-4-8(17)9(7)15(12)11(19)13(14,6-16)21-20-12/h2-4,8-9,16-17H,5-6H2,1H3/t8-,9-,12+,13+/m0/s1
|
|
| InChIKey |
FIVPIPIDMRVLAY-RBJBARPLSA-N
|
|
| Synonyms |
gliotoxin; 67-99-2; Aspergillin; Gliotoxin from Gliocladium fimbriatum; C13H14N2O4S2; CHEBI:5385; CHEMBL331627; 5L648PH06K; NSC 102866; (3R,5aS,6S,10aR)-6-hydroxy-3-(hydroxymethyl)-2-methyl-2,3,5a,6-tetrahydro-10H-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione; (3R,5aS,6S,10aR)-6-hydroxy-3-(hydroxymethyl)-2-methyl-2,3,6,10-tetrahydro-5aH-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione; CCRIS 4025; BRN 0050675; UNII-5L648PH06K; AI3-62383; NSC102866; S.N. 12870; NSC-102866; MFCD00058534; GLIOTOXIN [MI]; SCHEMBL54420; BSPBio_001237; 10H-3,10a-Epidithiopyrazino(1,2-a)indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-; 4-27-00-08902 (Beilstein Handbook Reference); DTXSID60877179; HMS1792M19; HMS1990M19; HMS3403M19; PI129; HY-N6727; ZINC3875454; BDBM50134315; CCG-35318; CCG-36058; AKOS024457211; QTL1_000040; NCGC00163466-01; NCGC00163466-02; 10H-3,10a-Epidithiopyrazino(1,2-a)indole-1,4-dione, 2,3,5a,6-hydroxy-3- (hydroxymethyl)-2-methyl-, (3R-(3-alpha,5a-beta,6-beta,10a-alpha))-; AC-31273; BG162731; CS-0029133; S. N.-12870; Q413364; SR-05000002340; SR-05000002340-2; (1R,7S,8S,11R)-7-hydroxy-11-(hydroxymethyl)-15-methyl-12,13-dithia-9,15-diazatetracyclo[9.2.2.01,9.03,8]pentadeca-3,5-diene-10,14-dione; (3R,5aS,6S,10aR)-2,3,5a,6-Tetrahydr o-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H-3,10a-e pidithiopyrazino[1,2-a]indole-1,4-dione; (3R,5aS,6S,10aR)-2,3,5a,6-Tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione; 10H-3,10A-EPIDITHIOPYRAZINO(1,2-A)INDOLE-1,4-DIONE, 2,3,5A,6-TETRAHYDRO-6-HYDROXY-3-(HYDROXYMETHYL)-2-METHYL-, (3R,5AS,6S,10AR)-; 2,3,5a,6-Tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H-3,10a-epidithiopyrazino[1,2-a]indole-1,4 dione; 6-Hydroxy-3-(hydroxymethyl)-2-methyl-2,3,6,10-tetrahydro-5aH-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione; 7-hydroxy-11-hydroxymethyl-12-methyl-14,15-dithia-9,12-diazatetracyclo[9.2.2.01,9.03,8]pentadeca-3,5-diene-10,13-dione
|
|
| CAS | 67-99-2 | |
| PubChem CID | 6223 | |
| ChEMBL ID | CHEMBL331627 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 326.4 | ALogp: | -0.7 |
| HBD: | 2 | HBA: | 6 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 132.0 | Aromatic Rings: | 5 |
| Heavy Atoms: | 21 | QED Weighted: | 0.667 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.027 | MDCK Permeability: | 0.00002250 |
| Pgp-inhibitor: | 0.013 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.164 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.071 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.15 | Plasma Protein Binding (PPB): | 57.80% |
| Volume Distribution (VD): | 0.623 | Fu: | 54.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.014 | CYP1A2-substrate: | 0.101 |
| CYP2C19-inhibitor: | 0.489 | CYP2C19-substrate: | 0.887 |
| CYP2C9-inhibitor: | 0.827 | CYP2C9-substrate: | 0.102 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.09 |
| CYP3A4-inhibitor: | 0.089 | CYP3A4-substrate: | 0.97 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.767 | Half-life (T1/2): | 0.703 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.16 |
| Drug-inuced Liver Injury (DILI): | 0.98 | AMES Toxicity: | 0.04 |
| Rat Oral Acute Toxicity: | 0.783 | Maximum Recommended Daily Dose: | 0.882 |
| Skin Sensitization: | 0.804 | Carcinogencity: | 0.908 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.008 |
| Respiratory Toxicity: | 0.605 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000993 | 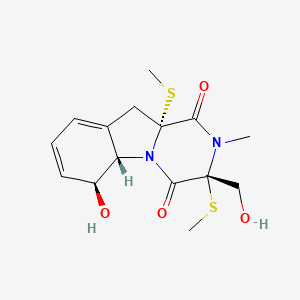 |
0.538 | D0Z8EX | 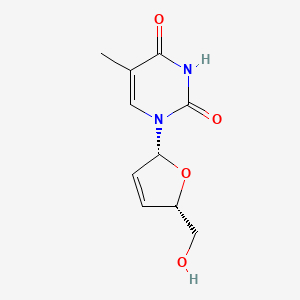 |
0.214 | ||
| ENC004752 | 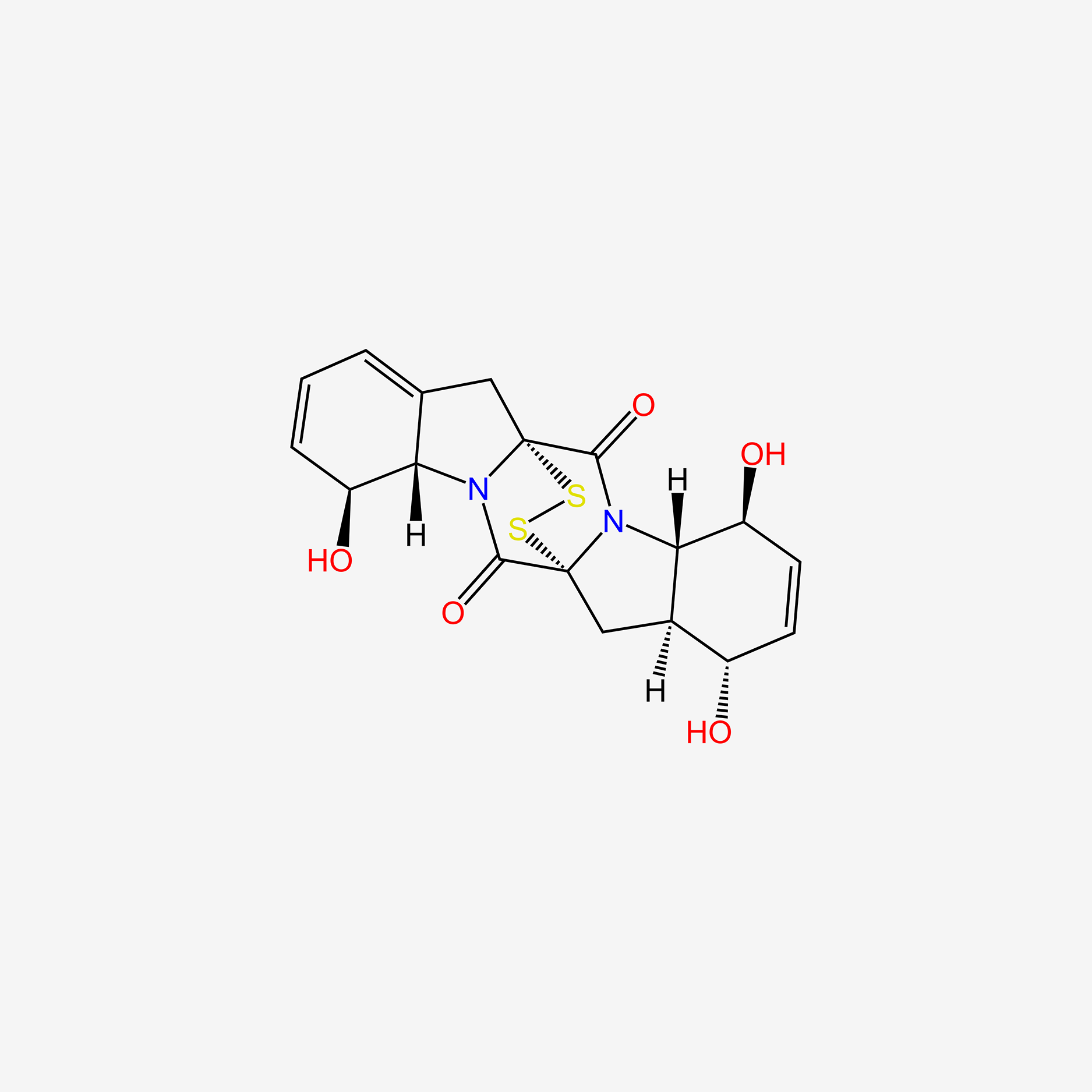 |
0.506 | D0CL9S | 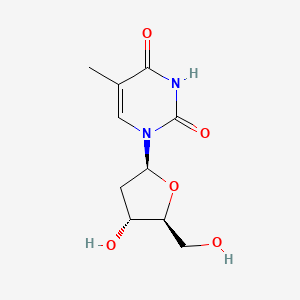 |
0.209 | ||
| ENC006009 | 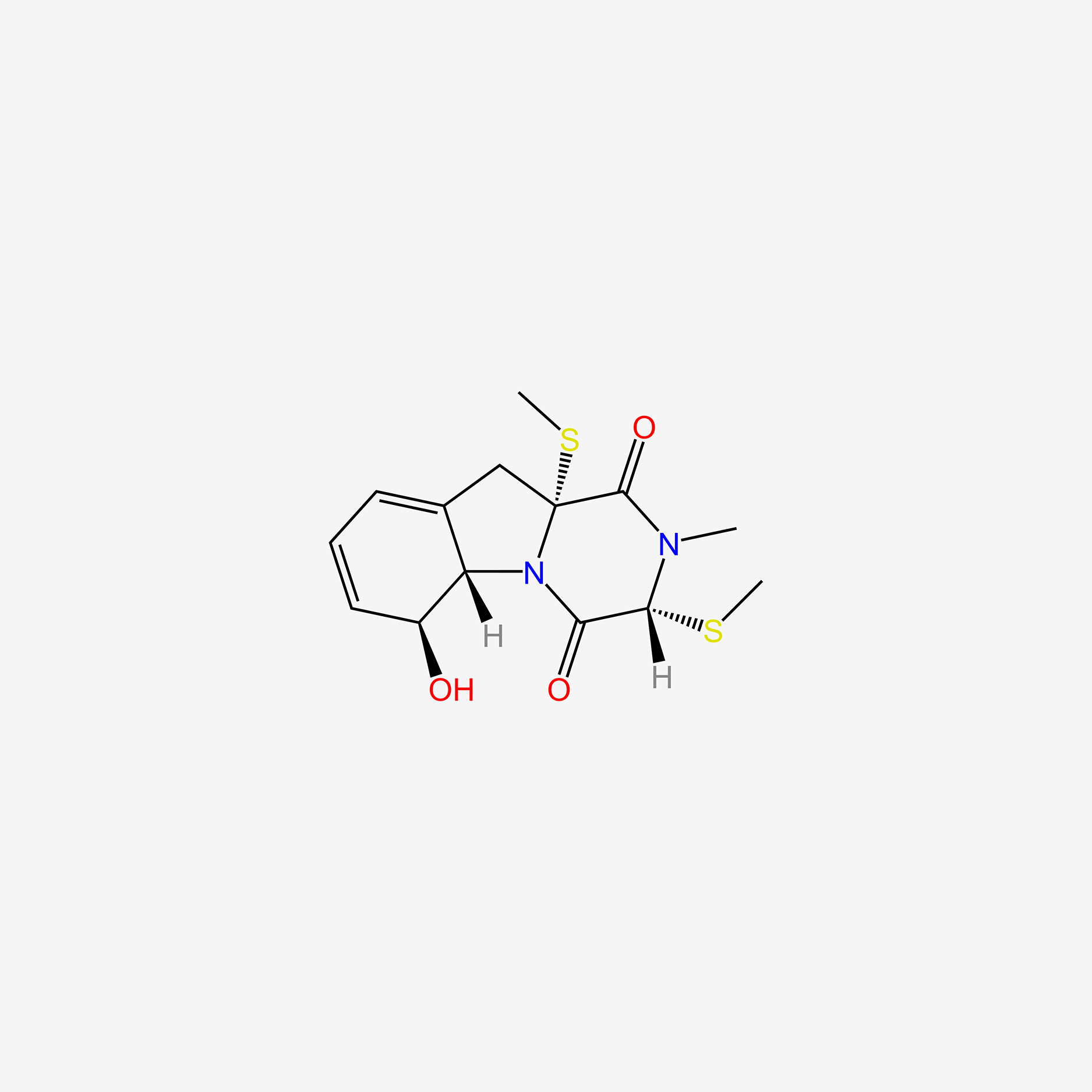 |
0.438 | D08EOD | 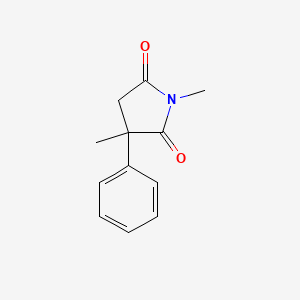 |
0.207 | ||
| ENC005509 | 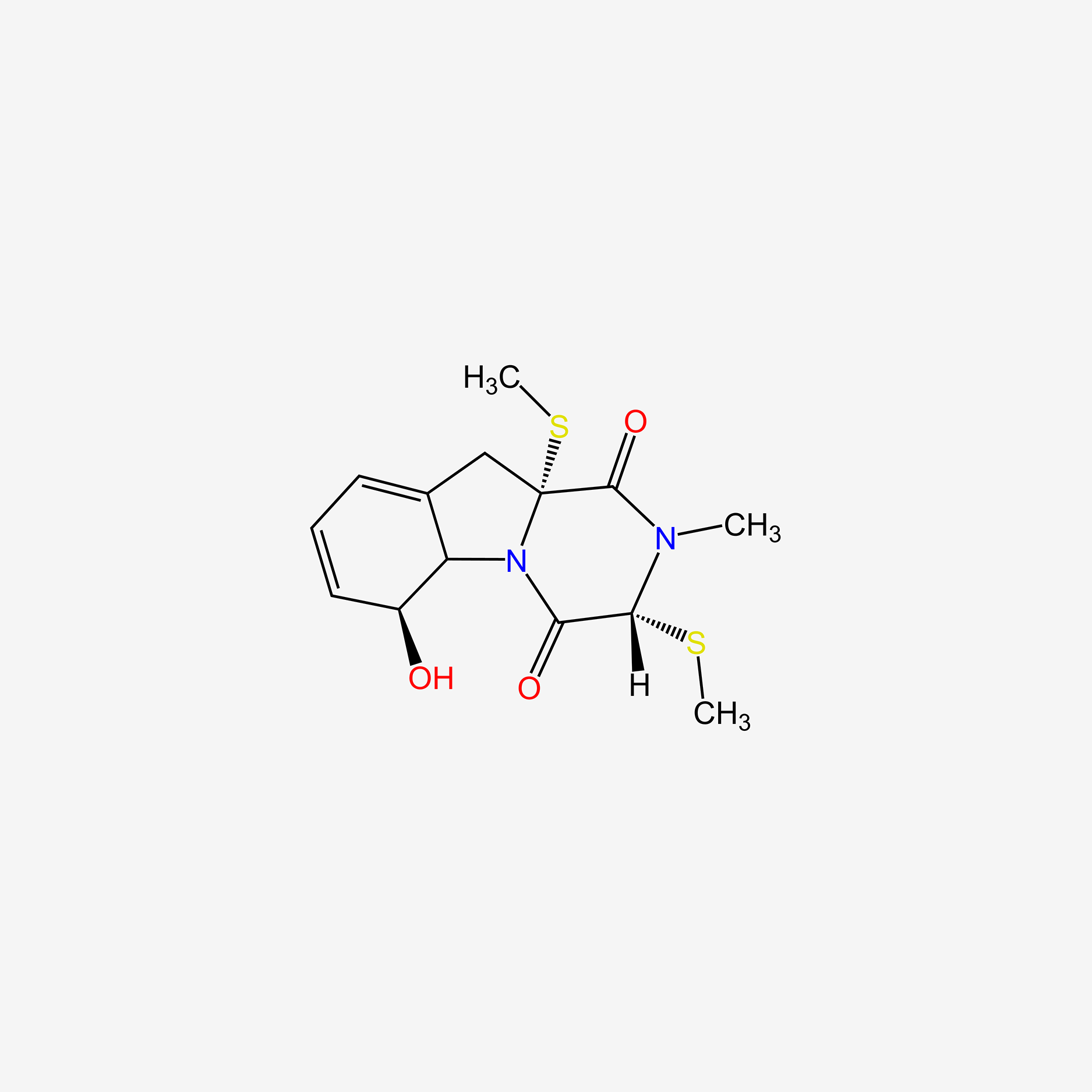 |
0.438 | D0R2KF | 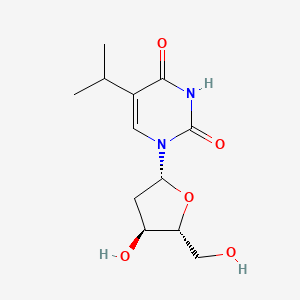 |
0.198 | ||
| ENC003617 | 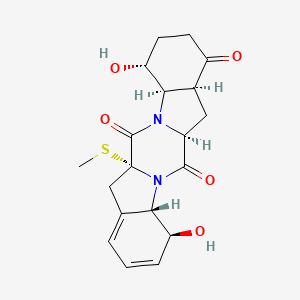 |
0.357 | D0TS1Z | 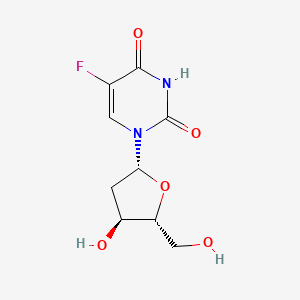 |
0.195 | ||
| ENC003438 | 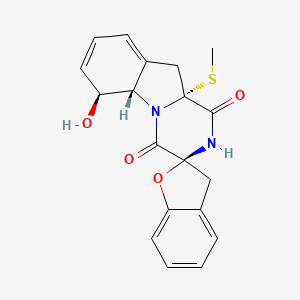 |
0.347 | D09PZO | 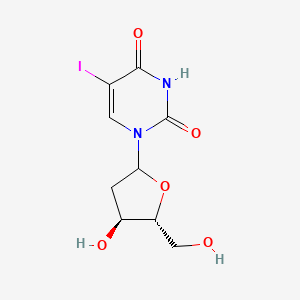 |
0.195 | ||
| ENC005392 | 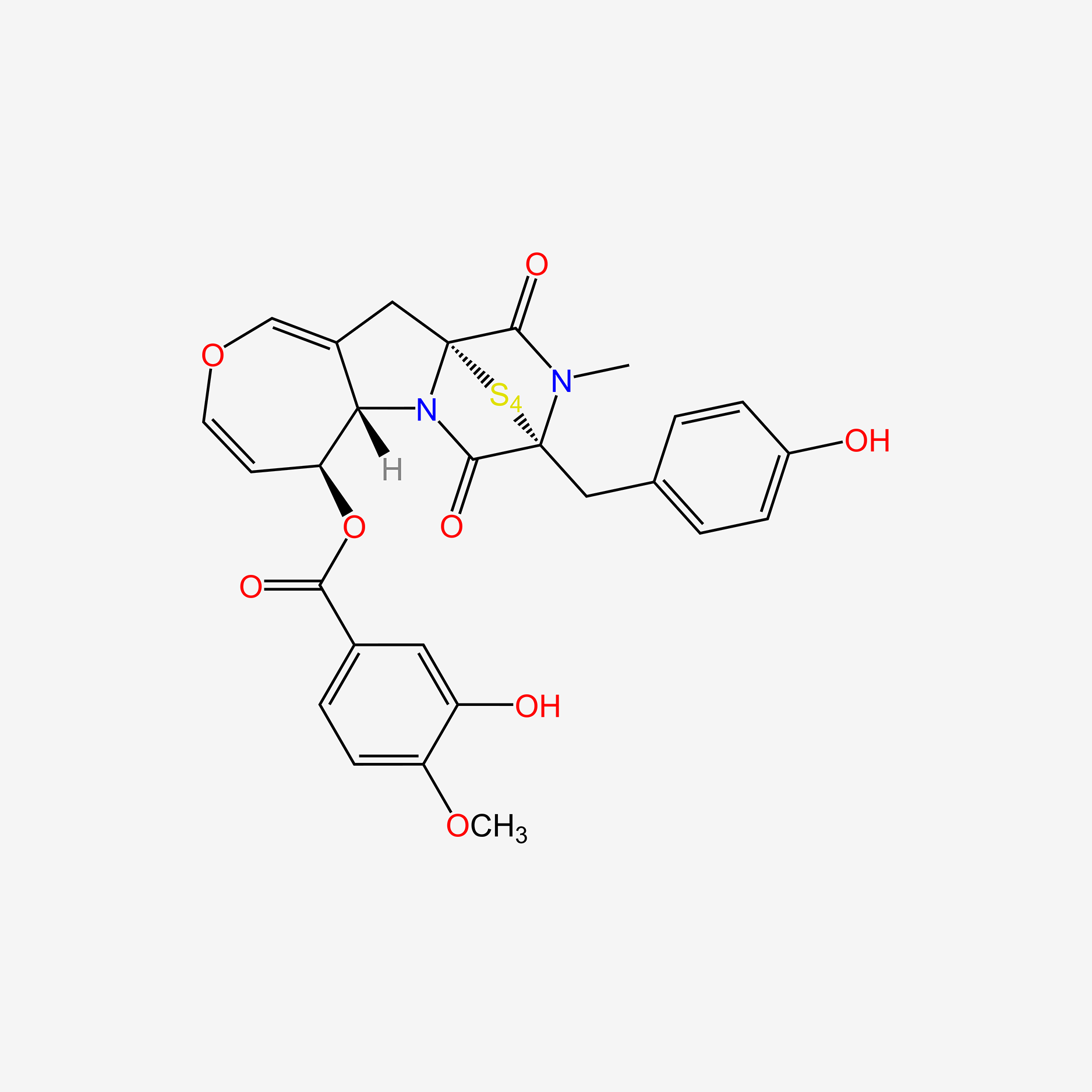 |
0.318 | D0IL7L | 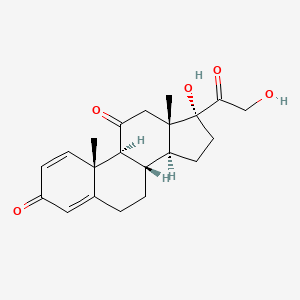 |
0.194 | ||
| ENC003809 | 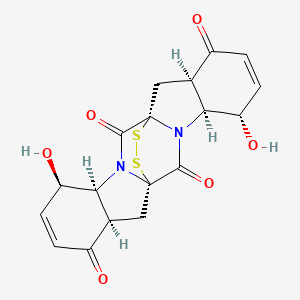 |
0.295 | D0I5DS |  |
0.191 | ||
| ENC003992 |  |
0.277 | D0W7RJ |  |
0.191 | ||
| ENC003595 |  |
0.277 | D0G6AB |  |
0.188 | ||