NPs Basic Information
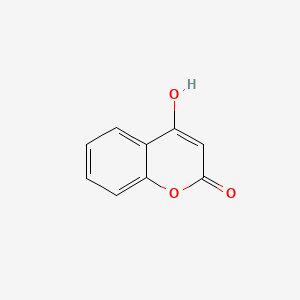
|
Name |
4-Hydroxycoumarin
|
| Molecular Formula | C9H6O3 | |
| IUPAC Name* |
4-hydroxychromen-2-one
|
|
| SMILES |
C1=CC=C2C(=C1)C(=CC(=O)O2)O
|
|
| InChI |
InChI=1S/C9H6O3/c10-7-5-9(11)12-8-4-2-1-3-6(7)8/h1-5,10H
|
|
| InChIKey |
VXIXUWQIVKSKSA-UHFFFAOYSA-N
|
|
| Synonyms |
4-Hydroxycoumarin; 1076-38-6; 4-Coumarinol; Benzotetronic acid; 4-Hydroxy-2H-chromen-2-one; 4-hydroxychromen-2-one; 2H-1-BENZOPYRAN-2-ONE, 4-HYDROXY-; Coumarin, 4-hydroxy-; 4-Hydroxy-2H-1-benzopyran-2-one; 4-Hydroxy coumarin; 4-Hydroxy-chromen-2-one; 4-HYDROXY-1-BENZOPYRAN-2-ONE; 4-hydroxy-2-chromenone; 2-Hydroxychromone; MFCD00006856; 4-hydroxy-2H-benzo[b]pyran-2-one; X954ZLL2RD; CHEMBL301141; 22105-09-5; CHEBI:40070; NSC11889; NSC-11889; 4-Hydroxy-2H-1-benzopyran-2-one (4-Hydroxycoumarin); 4-hydroxy-coumarin; 4- Hydroxycoumarin; 4HC; EINECS 214-060-2; NSC 11889; 4H-1-Benzopyran-4-one, 2-hydroxy-; UNII-X954ZLL2RD; BRN 0129768; hydroxychromone; AI3-52393; 4-hyroxycoumarin; 4-hydroxyl coumarin; 4-Monohydroxycoumarin; 4-Hydroxycoumarin, 98%; CBiol_000838; WLN: T66 BOVJ EQ; 5-18-01-00378 (Beilstein Handbook Reference); MLS004491719; SCHEMBL131312; MEGxm0_000452; SCHEMBL1961365; DTXSID8061472; ACon1_001952; 4-Hydroxy-2H-chromen-2-one #; DTXSID50944748; HMS1607G02; 4-hydroxy-2-oxo-2H-1-benzopyran; 2-Hydroxy-4H-1-benzopyran-4-one; BCP31120; HY-N6856; STR01861; BBL027616; BDBM50055710; ICCB4_000134; STK801816; ZINC18154848; ZINC96006086; AKOS000119142; AKOS037515220; AM84328; DB03410; 4-HYDROXYCOUMARIN [USP IMPURITY]; NCGC00179970-01; AC-13227; NCI60_000453; SMR000112320; SY001614; DB-014485; BB 0220623; CS-0015925; EU-0066799; FT-0602218; FT-0660645; H0235; EN300-17333; C20414; D71127; WARFARIN SODIUM IMPURITY B [EP IMPURITY]; 4-Hydroxycoumarin, Vetec(TM) reagent grade, 98%; SR-01000389319; J-515519; J-620003; SR-01000389319-1; BRD-K48844111-001-01-4; Q25323691; Z56922074; 4-HYDROXY-1-BENZOPYRAN-2-ONE; 4-HYDROXYCOUMARIN; F0266-2972; 4-Hydroxy Coumarin;4-Coumarinol;4-Hydroxy-2H-chromen-2-one
|
|
| CAS | 1076-38-6 | |
| PubChem CID | 54682930 | |
| ChEMBL ID | CHEMBL301141 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 162.14 | ALogp: | 1.3 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.723 | MDCK Permeability: | 0.00001760 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.961 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.996 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.123 | Plasma Protein Binding (PPB): | 87.71% |
| Volume Distribution (VD): | 0.525 | Fu: | 16.48% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.95 | CYP1A2-substrate: | 0.908 |
| CYP2C19-inhibitor: | 0.212 | CYP2C19-substrate: | 0.072 |
| CYP2C9-inhibitor: | 0.073 | CYP2C9-substrate: | 0.818 |
| CYP2D6-inhibitor: | 0.197 | CYP2D6-substrate: | 0.726 |
| CYP3A4-inhibitor: | 0.049 | CYP3A4-substrate: | 0.204 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.507 | Half-life (T1/2): | 0.83 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.089 | Human Hepatotoxicity (H-HT): | 0.095 |
| Drug-inuced Liver Injury (DILI): | 0.733 | AMES Toxicity: | 0.116 |
| Rat Oral Acute Toxicity: | 0.404 | Maximum Recommended Daily Dose: | 0.327 |
| Skin Sensitization: | 0.534 | Carcinogencity: | 0.786 |
| Eye Corrosion: | 0.657 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.433 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002809 | 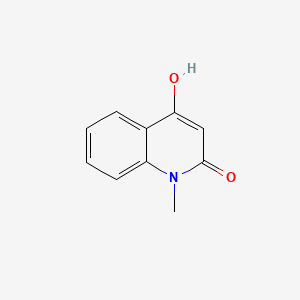 |
0.522 | D0QV5T | 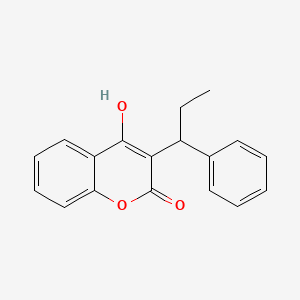 |
0.382 | ||
| ENC002793 | 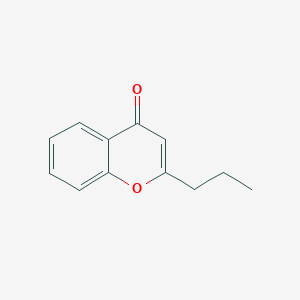 |
0.510 | D0Z3DY | 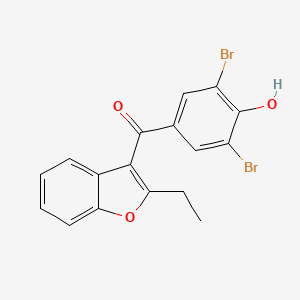 |
0.377 | ||
| ENC000025 | 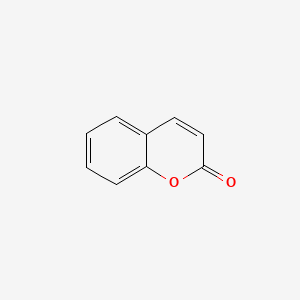 |
0.500 | D0E3OF | 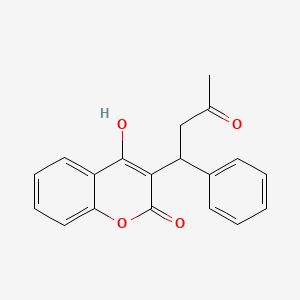 |
0.356 | ||
| ENC001539 | 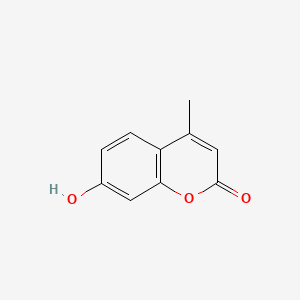 |
0.458 | D07HBX |  |
0.356 | ||
| ENC001562 | 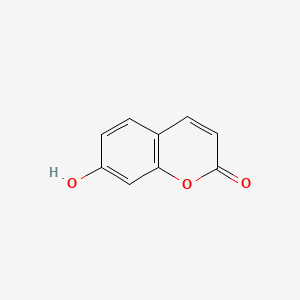 |
0.447 | D09ZIS | 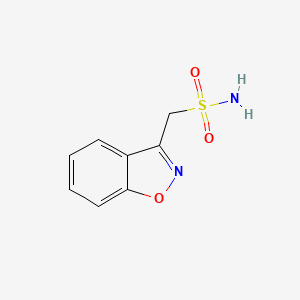 |
0.352 | ||
| ENC000036 | 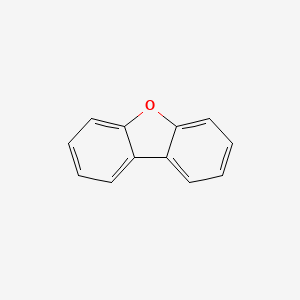 |
0.431 | D02TJS | 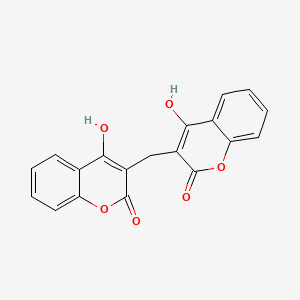 |
0.329 | ||
| ENC001561 | 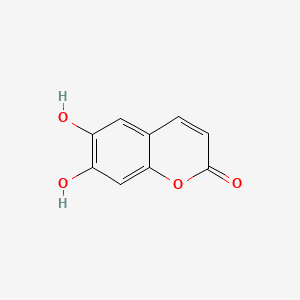 |
0.429 | D05HFY | 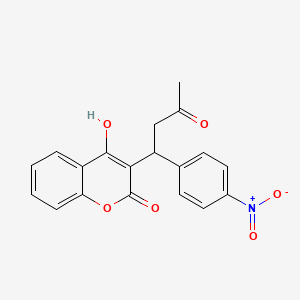 |
0.325 | ||
| ENC001447 | 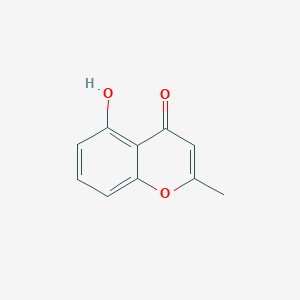 |
0.429 | D0L5PO | 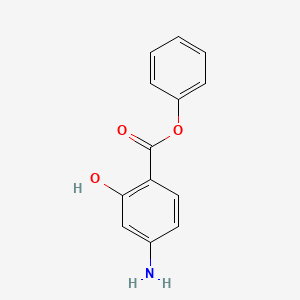 |
0.323 | ||
| ENC000675 | 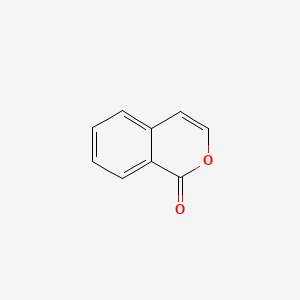 |
0.404 | D03GET | 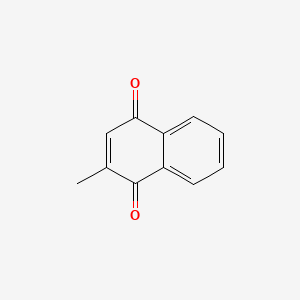 |
0.321 | ||
| ENC001537 | 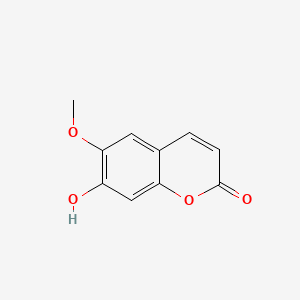 |
0.404 | D0D5GG | 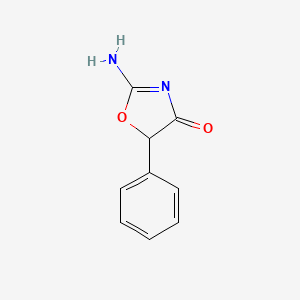 |
0.315 | ||