NPs Basic Information
|
Name |
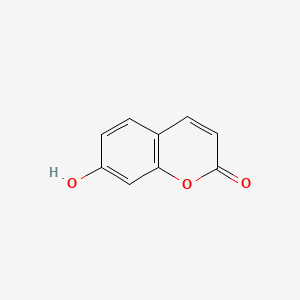
Umbelliferone
|
| Molecular Formula | C9H6O3 | |
| IUPAC Name* |
7-hydroxychromen-2-one
|
|
| SMILES |
C1=CC(=CC2=C1C=CC(=O)O2)O
|
|
| InChI |
InChI=1S/C9H6O3/c10-7-3-1-6-2-4-9(11)12-8(6)5-7/h1-5,10H
|
|
| InChIKey |
ORHBXUUXSCNDEV-UHFFFAOYSA-N
|
|
| Synonyms |
Umbelliferone; 7-hydroxycoumarin; 93-35-6; 7-Hydroxy-2H-chromen-2-one; Hydrangin; Skimmetin; 7-hydroxycoumarine; Hydrangine; Skimmetine; 7-Oxycoumarin; Umbelliferon; 2H-1-Benzopyran-2-one, 7-hydroxy-; 7-Hydroxy-2H-1-benzopyran-2-one; 7-hydroxychromen-2-one; Coumarin, 7-hydroxy-; beta-Umbelliferone; 7-hydroxy coumarin; NSC 19790; 7 HC; 7-hydroxy-coumarin; MFCD00006878; NSC19790; CHEMBL51628; 60Z60NTL4G; 7-HC; 7-hydroxy-1-benzopyran-2-one; CHEBI:27510; NSC-19790; 7-oxidanylchromen-2-one; 7-hydroxycumarin; Dichrin A; CCRIS 3591; EINECS 202-240-3; 32922-68-2; BRN 0127683; UNII-60Z60NTL4G; AI3-38054; 7-hydroxycoumarin, 14C-labeled; 7-hydroxycournarin; beta -umbelliferone; 07L; 7-hydroxy-coumarine; .beta.-Umbelliferone; Umbelliferone, 99%; 7-Hydroxy-2-chromenone; Coumarin derivative, 3a; 7 OH COUMARIN; Spectrum2_001962; Spectrum3_000751; 7-hydroxy-chromen-2-one; 7-hydroxycoumarin sulphate; UMBELLIFERONE [MI]; 7-Hydroxy Coumarin ,(S); BIDD:PXR0126; SCHEMBL22018; BSPBio_002362; SPECTRUM231084; 5-18-01-00386 (Beilstein Handbook Reference); MLS002207035; BIDD:ER0671; 2-Hydroxy-7H-chromen-7-one; SPBio_002083; 7-Hydroxy Coumarin-[13C6]; UMBELLIFERONE [WHO-DD]; MEGxp0_000814; DTXSID5052626; ACon1_000219; KBio3_001582; ZINC58111; 7-Hydroxy-2H-chromen-2-one #; Umbelliferone, analytical standard; HMS1607M21; HMS2271N09; HMS3741M03; 2-Hydroxy-7H-1-benzopyran-7-one; ALBB-021296; CS-D1186; HY-N0573; STR04824; BBL027620; BDBM50174558; CCG-39436; s3675; STK331042; Umbelliferone (Hydrangin, Skimmetin); AKOS000120867; NSC-019790; SDCCGMLS-0066941.P001; 7-Hydroxycoumarin;Hydrangin;NSC 19790; NCGC00095801-01; NCGC00095801-02; NCGC00095801-03; NCGC00178691-01; NCGC00178691-02; AC-18399; AC-34707; NCI60_001646; SMR000112324; SY001924; DB-014673; BB 0218364; FT-0621430; H0236; EN300-18075; Umbelliferone, Vetec(TM) reagent grade, 98%; A14827; C09315; U-3000; A801734; A844525; Q416196; BRD-K87991767-001-02-0; BRD-K87991767-001-03-8; Z57150899; F0722-0129; 7-Hydroxycoumarin; Umbelliferone; 7-Hydroxy-2H-1-benzopyran-2-one; Umbelliferone, suitable for fluorescence indicator, >=98.0% (HPLC); 142044-47-1
|
|
| CAS | 93-35-6 | |
| PubChem CID | 5281426 | |
| ChEMBL ID | CHEMBL51628 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 162.14 | ALogp: | 1.6 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.679 | MDCK Permeability: | 0.00001480 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.986 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.991 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.08 | Plasma Protein Binding (PPB): | 85.68% |
| Volume Distribution (VD): | 0.603 | Fu: | 18.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.973 | CYP1A2-substrate: | 0.847 |
| CYP2C19-inhibitor: | 0.282 | CYP2C19-substrate: | 0.063 |
| CYP2C9-inhibitor: | 0.041 | CYP2C9-substrate: | 0.918 |
| CYP2D6-inhibitor: | 0.664 | CYP2D6-substrate: | 0.859 |
| CYP3A4-inhibitor: | 0.603 | CYP3A4-substrate: | 0.231 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.575 | Half-life (T1/2): | 0.834 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.101 | Human Hepatotoxicity (H-HT): | 0.121 |
| Drug-inuced Liver Injury (DILI): | 0.723 | AMES Toxicity: | 0.047 |
| Rat Oral Acute Toxicity: | 0.378 | Maximum Recommended Daily Dose: | 0.191 |
| Skin Sensitization: | 0.428 | Carcinogencity: | 0.841 |
| Eye Corrosion: | 0.575 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.17 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000025 | 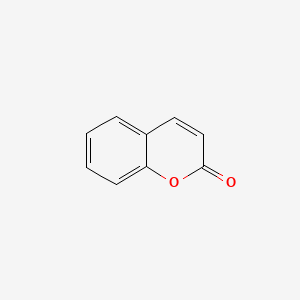 |
0.571 | D08SKH | 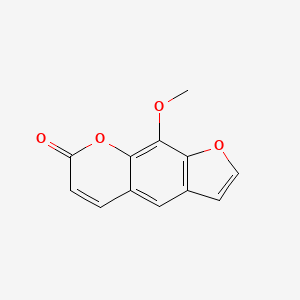 |
0.379 | ||
| ENC001561 | 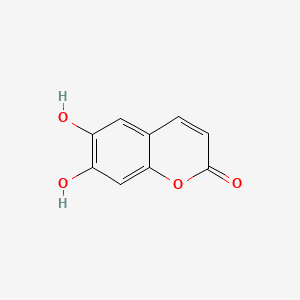 |
0.556 | D03UOT |  |
0.333 | ||
| ENC001539 | 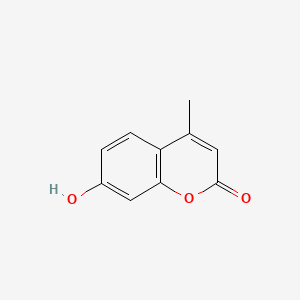 |
0.522 | D0S2BV | 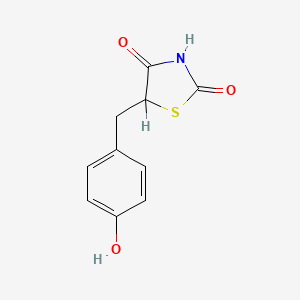 |
0.310 | ||
| ENC001537 | 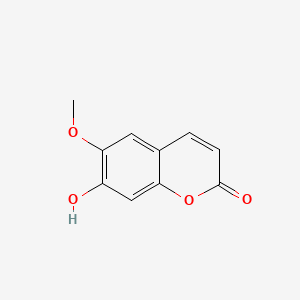 |
0.521 | D0DJ1B |  |
0.306 | ||
| ENC002806 | 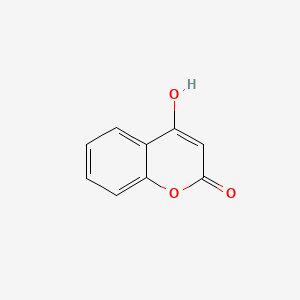 |
0.447 | D0U5QK | 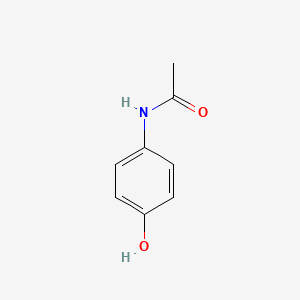 |
0.306 | ||
| ENC002764 | 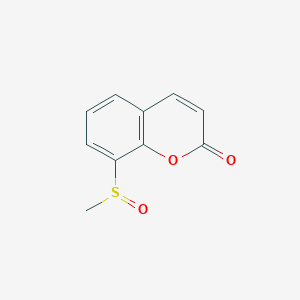 |
0.431 | D08ZEB | 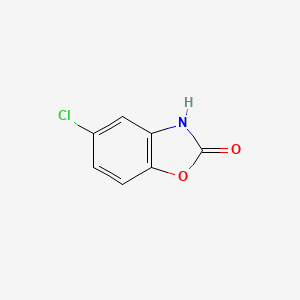 |
0.300 | ||
| ENC004623 | 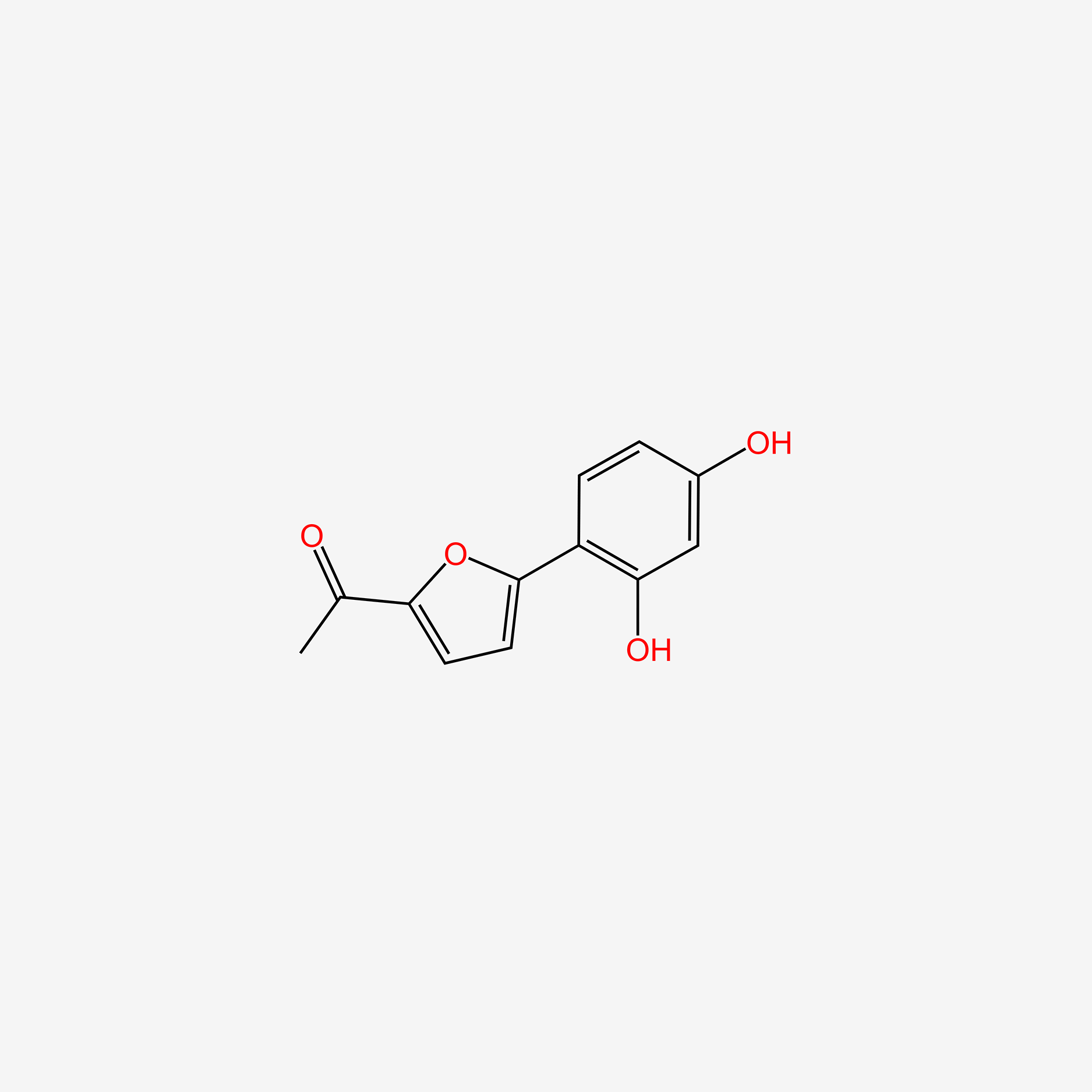 |
0.393 | D04AIT |  |
0.296 | ||
| ENC001524 | 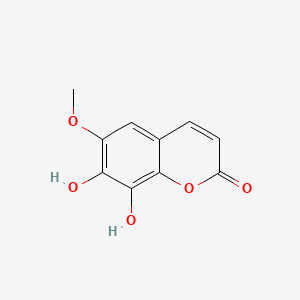 |
0.389 | D0K8KX |  |
0.288 | ||
| ENC000078 | 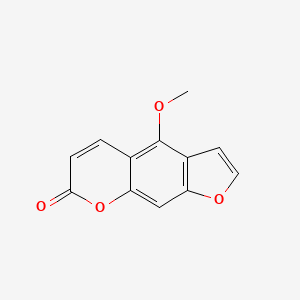 |
0.379 | D05CKR | 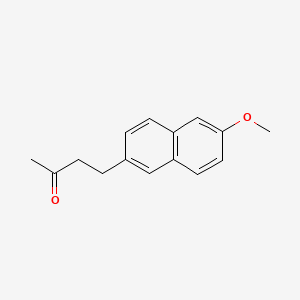 |
0.281 | ||
| ENC001576 | 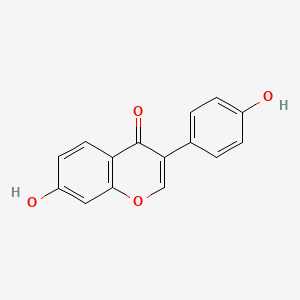 |
0.375 | D01CRB | 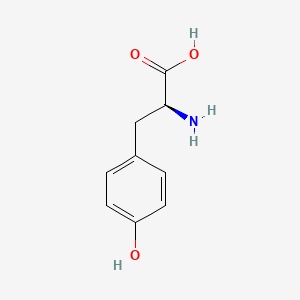 |
0.278 | ||