NPs Basic Information
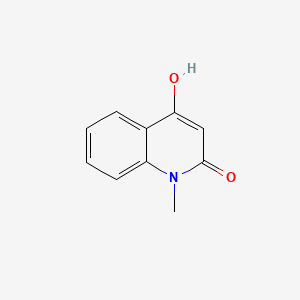
|
Name |
4-Hydroxy-1-methyl-2-quinolone
|
| Molecular Formula | C10H9NO2 | |
| IUPAC Name* |
4-hydroxy-1-methylquinolin-2-one
|
|
| SMILES |
CN1C2=CC=CC=C2C(=CC1=O)O
|
|
| InChI |
InChI=1S/C10H9NO2/c1-11-8-5-3-2-4-7(8)9(12)6-10(11)13/h2-6,12H,1H3
|
|
| InChIKey |
RTNPPPQVXREFKX-UHFFFAOYSA-N
|
|
| Synonyms |
4-Hydroxy-1-methyl-2-quinolone; 1677-46-9; 4-Hydroxy-1-methyl-2(1H)-quinolone; 4-hydroxy-1-methylquinolin-2(1H)-one; 4-Hydroxy-1-methylcarbostyril; 1-Methyl-4-hydroxycarbostyril; 2(1H)-Quinolinone, 4-hydroxy-1-methyl-; 4-Hydroxy-N-methylcarbostyril; 4-Hydroxy-1-methyl-2(1H)-quinolinone; CARBOSTYRIL, 4-HYDROXY-1-METHYL-; 1-Methyl-4-hydroxy-2-chinolon; 4-hydroxy-1-methylquinolin-2-one; N-Methyl-4-hydroxykarbostyril; 1-Methyl-4-hydroxy-2-chinolinon; NSC 39973; NSC39973; N-Methyl-4-hydroxykarbostyril [Czech]; 1-Methyl-4-hydroxy-2-chinolon [Czech]; V713CAW761; MFCD00024052; NSC-39973; 1-methyl-4-hydroxy-2(1H)-quinolinone; 4-HYDROXY-1-METHYL-1H-2-QUINOLINONE; N-Methyl-4-hydroxykarbostyril (CZECH); 1-Methyl-4-hydroxy-2-chinolon (CZECH); 4-hydroxy-1-methyl-1,2-dihydroquinolin-2-one; EINECS 216-830-3; 4-Hydroxy-1-methyl-1H-quinolin-2-one; 4-HYDROXY-N-METHYL-2-QUINOLONE; BRN 1528990; 1-Methyl-4-hydroxy-2-chinolinon [Czech]; 2(1H)-Quinolinone,4-hydroxy-1-methyl-; Maybridge1_002182; Oprea1_834130; 5-21-12-00311 (Beilstein Handbook Reference); SCHEMBL835056; UNII-V713CAW761; 4-Hydroxy-1-methyl carbostyril; CHEMBL2022051; DTXSID0061874; SCHEMBL13143143; 1-methyl-4-hydroxy-2-quinolone; HMS547L04; WLN: T66 BNVJ B1 EQ; n-methyl-4-hydroxy-2-quinolinone; 4-hydroxy-1-methyl-2-quinolinone; 4-hydroxy-1-methyl-quinolin-2-one; ALBB-019832; BBL036914; STK327478; STL514059; ZINC18190627; AKOS000121455; AKOS037491726; ZINC103644054; SDCCGMLS-0065887.P001; 1-methyl-4-hydroxyquinolin-2(1H)-one; 2-hydroxy-1-methylquinolin-4(1H)-one; N-METHYL-4-HYDROXYQUINOL-2-ONE; UPCMLD0ENAT5889157:001; 1-METHYL-4-HYDROXYQUINOLIN-2-ONE; 4-HYDROXY-N-METHYL-2-QUINOLINONE; NS-01441; DB-043712; CS-0131691; EU-0087440; FT-0618613; M1009; 4-Hydroxy-1-methyl-2(1H)-quinolone, 98%; 4-HYDROXY-1-METHYLQUINOLINE-2(1H)-ONE; AB00694265-02; A810900; W-109693; 1-METHYL-2-OXO-1,2-DIHYDRO-4-HYDROXYQUINOLINE
|
|
| CAS | 1677-46-9 | |
| PubChem CID | 54686436 | |
| ChEMBL ID | CHEMBL2022051 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 175.18 | ALogp: | 0.9 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 13 | QED Weighted: | 0.662 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.763 | MDCK Permeability: | 0.00002330 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.011 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.013 |
| 30% Bioavailability (F30%): | 0.005 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.905 | Plasma Protein Binding (PPB): | 83.44% |
| Volume Distribution (VD): | 0.858 | Fu: | 10.49% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.961 | CYP1A2-substrate: | 0.936 |
| CYP2C19-inhibitor: | 0.185 | CYP2C19-substrate: | 0.542 |
| CYP2C9-inhibitor: | 0.076 | CYP2C9-substrate: | 0.813 |
| CYP2D6-inhibitor: | 0.034 | CYP2D6-substrate: | 0.821 |
| CYP3A4-inhibitor: | 0.037 | CYP3A4-substrate: | 0.25 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.65 | Half-life (T1/2): | 0.642 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.089 |
| Drug-inuced Liver Injury (DILI): | 0.11 | AMES Toxicity: | 0.792 |
| Rat Oral Acute Toxicity: | 0.076 | Maximum Recommended Daily Dose: | 0.322 |
| Skin Sensitization: | 0.625 | Carcinogencity: | 0.851 |
| Eye Corrosion: | 0.044 | Eye Irritation: | 0.959 |
| Respiratory Toxicity: | 0.799 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002806 | 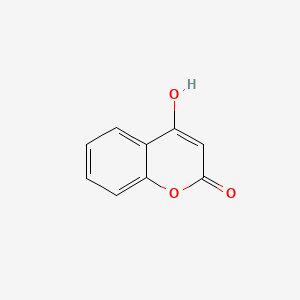 |
0.522 | D06GKN |  |
0.356 | ||
| ENC002158 |  |
0.431 | D0K7WK | 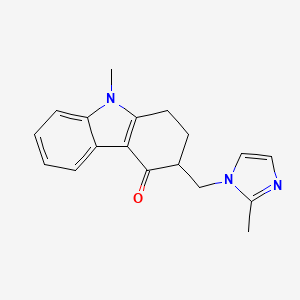 |
0.356 | ||
| ENC001042 | 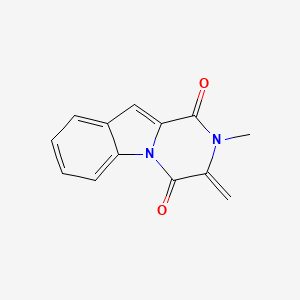 |
0.407 | D07HBX |  |
0.340 | ||
| ENC004686 | 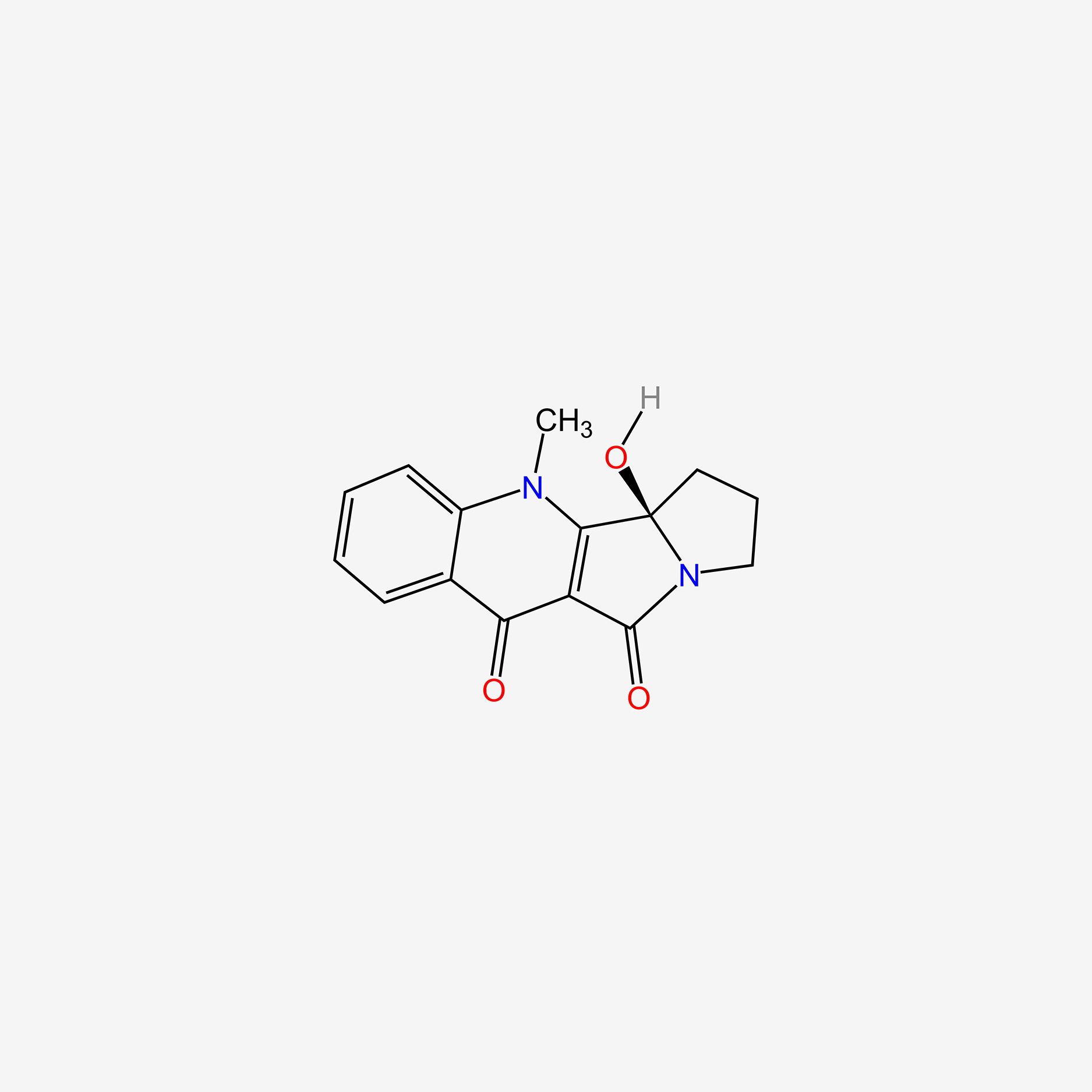 |
0.394 | D06BYV | 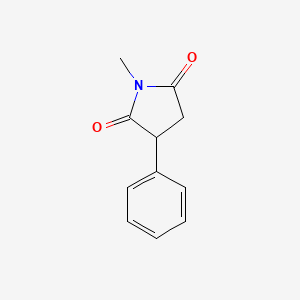 |
0.339 | ||
| ENC004693 | 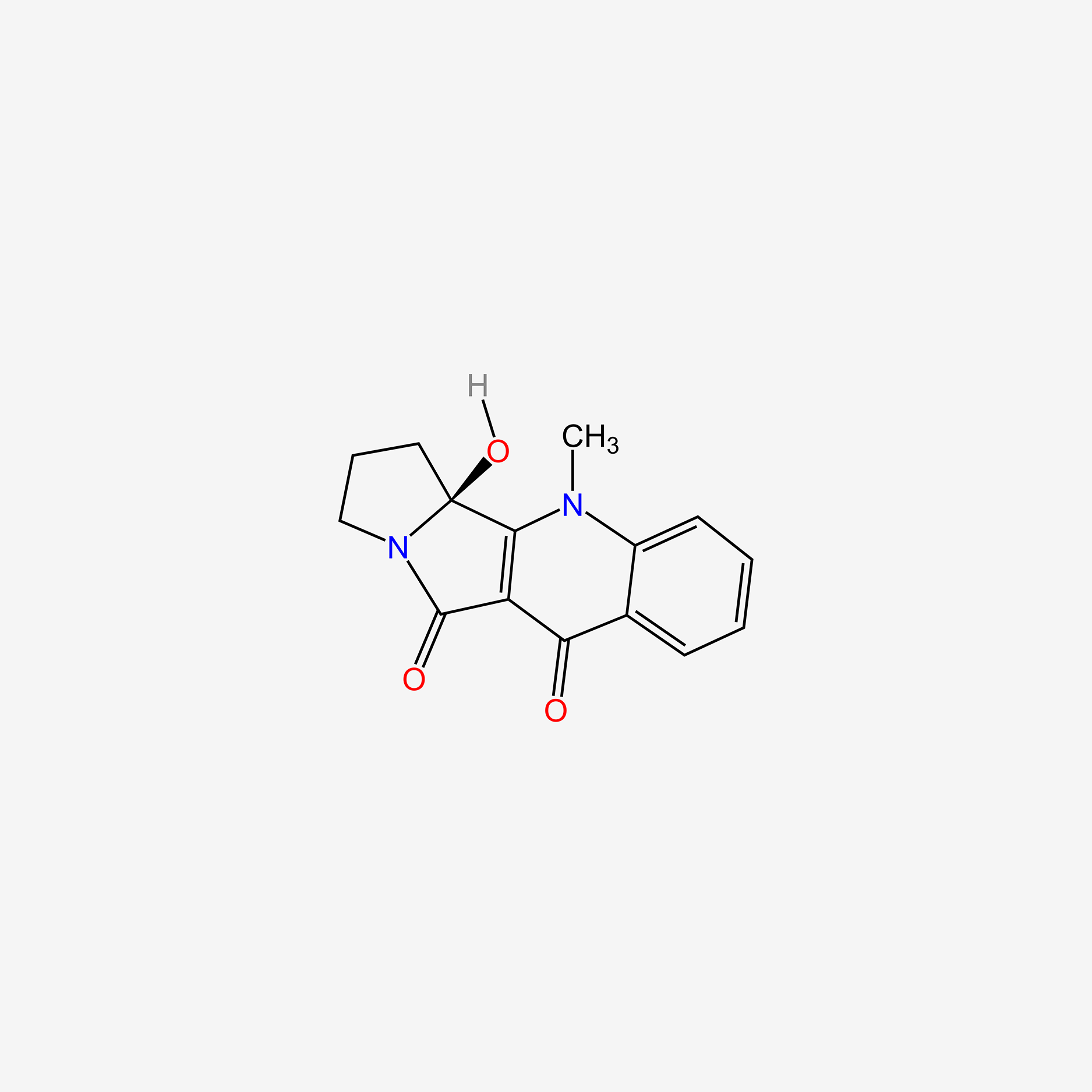 |
0.394 | D02WCI | 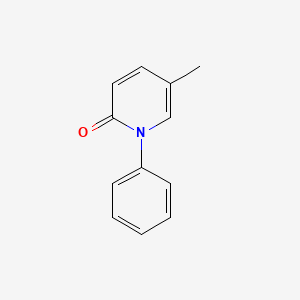 |
0.333 | ||
| ENC004684 |  |
0.382 | D03GET | 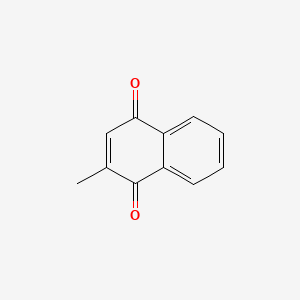 |
0.333 | ||
| ENC002793 | 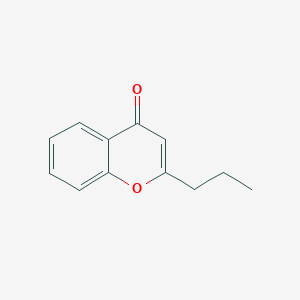 |
0.382 | D08EOD | 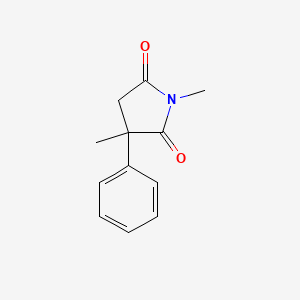 |
0.328 | ||
| ENC000028 | 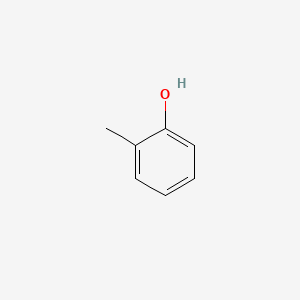 |
0.381 | D06DLI | 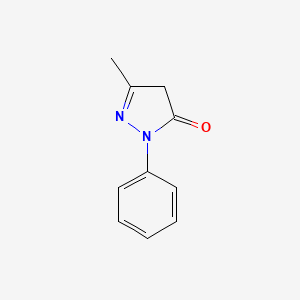 |
0.327 | ||
| ENC002566 |  |
0.379 | D06IXT | 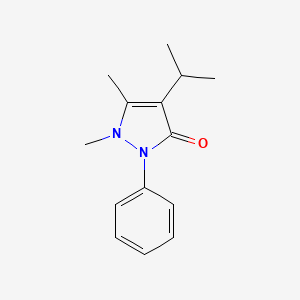 |
0.323 | ||
| ENC001367 | 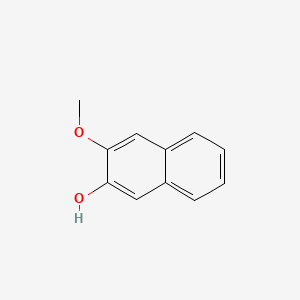 |
0.377 | D0B1FE | 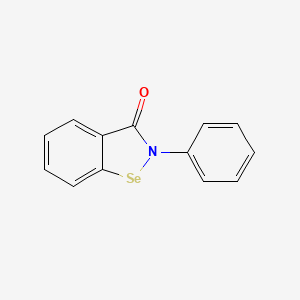 |
0.317 | ||