NPs Basic Information
|
Name |
Cryptochlorogenic acid
|
| Molecular Formula | C16H18O9 | |
| IUPAC Name* |
(3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxycyclohexane-1-carboxylic acid
|
|
| SMILES |
C1[C@H](C([C@@H](CC1(C(=O)O)O)O)OC(=O)/C=C/C2=CC(=C(C=C2)O)O)O
|
|
| InChI |
InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(21)25-14-11(19)6-16(24,15(22)23)7-12(14)20/h1-5,11-12,14,17-20,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14?,16?/m1/s1
|
|
| InChIKey |
GYFFKZTYYAFCTR-AVXJPILUSA-N
|
|
| Synonyms |
Cryptochlorogenic acid; 905-99-7; 4-Caffeoylquinic acid; 4-O-Caffeoylquinic acid; 4-Cqa; 4-o-Caffeoyl quinic acid; Quinic acid 4-O-caffeate; 4-O-trans-caffeoylquinic acid; 4-O-(3,4-Dihydroxycinnamoyl)-D-quinic acid; F23DJ84IZ9; (3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxycyclohexane-1-carboxylic acid; CHEMBL4203706; 4-(3,4-Dihydroxycinnamoyl)quinic acid; 82638-23-1; 87099-73-8; rel-(1S,3R,4S,5R)-4-(((E)-3-(3,4-Dihydroxyphenyl)acryloyl)oxy)-1,3,5-trihydroxycyclohexanecarboxylic acid; MFCD10566638; Cryptochlorogenic-acid; Quinic acid, 4-caffeoyl-; UNII-F23DJ84IZ9; 4-O-(E)-caffeoylquinic acid; CHEMBL3092676; Cinnamic acid, 3,4-dihydroxy-, 4-carboxy-2,4,6-trihydroxycyclohexyl ester; Quinic acid, 4-caffeoyl-, E-; SCHEMBL18180782; SCHEMBL20883249; ACon1_000120; CHEBI:75491; HY-N0787; BDBM50455380; s9319; ZINC31154929; 4-O-Caffeoylquinic acid, >=98.0%; AKOS037514601; ZINC100038257; ZINC103240311; CCG-268076; CS-3767; NCGC00180861-01; (1alpha,3alpha,4alpha,5beta)-4-(3-(3,4-(Dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,3,5-trihydroxycyclohexanecarboxylic acid; (3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxy-cyclohexanecarboxylic acid; BS-17821; Cryptochlorogenic acid, analytical standard; 4-Caffeoylquinic acid/ Cryptochlorogenic acid; 905C997; Q-100886; B0B55D52-5101-4E6D-AA67-72D1CECBAA5A; Q27145347; 49B68A4C-6EC9-4441-B7D7-E3B740A8CDEC; (1alpha,3R,4alpha,5R)-4-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy]-1,3,5-trihydroxycyclohexanecarboxylic acid; (1S,3R,4S,5R)-4-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,3,5-trihydroxycyclohexanecarboxylic acid; 1,3beta,5alpha-Trihydroxy-4alpha-(3,4-dihydroxycinnamoyloxy)cyclohexane-1beta-carboxylic acid; CYCLOHEXANECARBOXYLIC ACID, 4-(((2E)-3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPEN-1-YL)OXY)-1,3,5-TRIHYDROXY-, (1.ALPHA.,3R,4.ALPHA.,5R)-; Cyclohexanecarboxylic acid, 4-(((2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-1R-propenyl)oxy)-1,3,5-trihydroxy-, (1R-(1alpha,3alpha,4alpha,5beta))-; CYCLOHEXANECARBOXYLIC ACID, 4-((3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPEN-1-YL)OXY)-1,3,5-TRIHYDROXY-, (1.ALPHA.,3R,4.ALPHA.,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-; NCGC00180861-02!(3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxycyclohexane-1-carboxylic acid
|
|
| CAS | 905-99-7 | |
| PubChem CID | 9798666 | |
| ChEMBL ID | CHEMBL4203706 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 354.31 | ALogp: | -0.4 |
| HBD: | 6 | HBA: | 9 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 165.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 25 | QED Weighted: | 0.244 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.451 | MDCK Permeability: | 0.00010925 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.978 |
| Human Intestinal Absorption (HIA): | 0.864 | 20% Bioavailability (F20%): | 0.981 |
| 30% Bioavailability (F30%): | 0.997 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.732 | Plasma Protein Binding (PPB): | 44.12% |
| Volume Distribution (VD): | 0.48 | Fu: | 49.69% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.169 | CYP1A2-substrate: | 0.031 |
| CYP2C19-inhibitor: | 0.017 | CYP2C19-substrate: | 0.048 |
| CYP2C9-inhibitor: | 0.016 | CYP2C9-substrate: | 0.107 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.121 |
| CYP3A4-inhibitor: | 0.082 | CYP3A4-substrate: | 0.047 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.828 | Half-life (T1/2): | 0.914 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.036 | Human Hepatotoxicity (H-HT): | 0.234 |
| Drug-inuced Liver Injury (DILI): | 0.088 | AMES Toxicity: | 0.031 |
| Rat Oral Acute Toxicity: | 0.081 | Maximum Recommended Daily Dose: | 0.46 |
| Skin Sensitization: | 0.557 | Carcinogencity: | 0.048 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.05 |
| Respiratory Toxicity: | 0.081 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
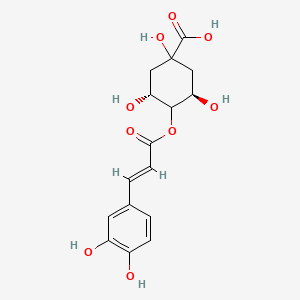
| ENC001471 |  |
0.811 | D0KN2M | 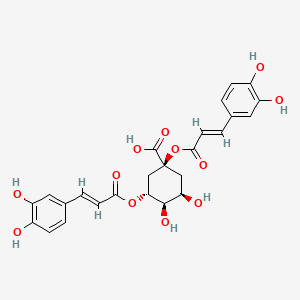 |
0.487 | ||
| ENC001543 | 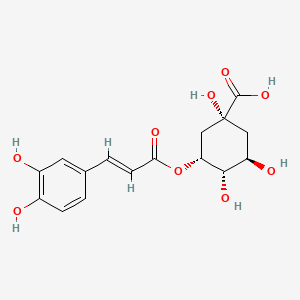 |
0.811 | D0V9EN |  |
0.457 | ||
| ENC001440 |  |
0.457 | D0I3RO |  |
0.293 | ||
| ENC001579 |  |
0.408 | D08HVR |  |
0.284 | ||
| ENC002582 |  |
0.361 | D0BA6T |  |
0.277 | ||
| ENC001101 |  |
0.346 | D0P7JZ |  |
0.267 | ||
| ENC004024 |  |
0.345 | D0U0OT | 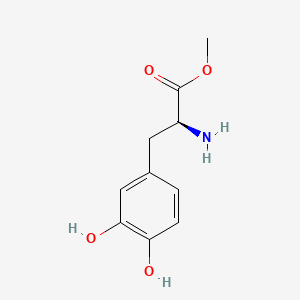 |
0.259 | ||
| ENC002823 | 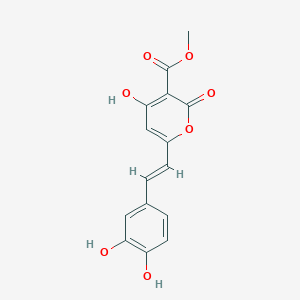 |
0.323 | D00KRE | 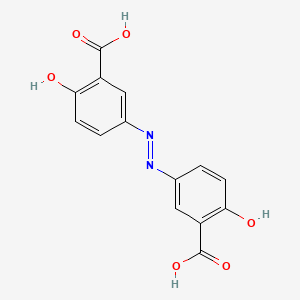 |
0.245 | ||
| ENC000002 | 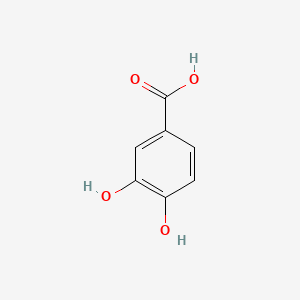 |
0.315 | D0Y6KO | 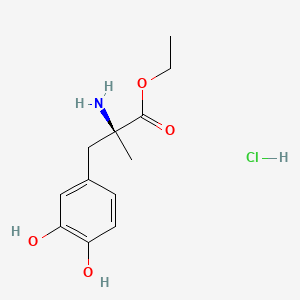 |
0.242 | ||
| ENC000035 | 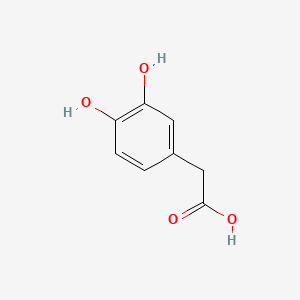 |
0.303 | D0E6OC | 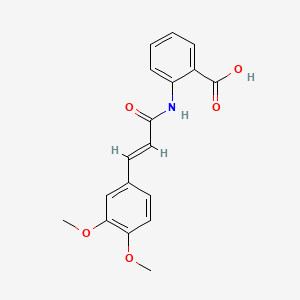 |
0.241 | ||