NPs Basic Information
|
Name |
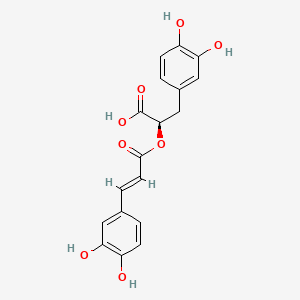
Rosmarinic acid
|
| Molecular Formula | C18H16O8 | |
| IUPAC Name* |
(2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoic acid
|
|
| SMILES |
C1=CC(=C(C=C1C[C@H](C(=O)O)OC(=O)/C=C/C2=CC(=C(C=C2)O)O)O)O
|
|
| InChI |
InChI=1S/C18H16O8/c19-12-4-1-10(7-14(12)21)3-6-17(23)26-16(18(24)25)9-11-2-5-13(20)15(22)8-11/h1-8,16,19-22H,9H2,(H,24,25)/b6-3+/t16-/m1/s1
|
|
| InChIKey |
DOUMFZQKYFQNTF-WUTVXBCWSA-N
|
|
| Synonyms |
rosmarinic acid; 20283-92-5; Rosemary acid; Rosmarinicacid; (R)-rosmarinic acid; Labiatic acid; Labiatenic acid; trans-Rosmarinic acid; Rosmarinate; Rosmarinic acid racemate; Rosmarinic-acid; 537-15-5; (R,E)-3-(3,4-dihydroxyphenyl)-2-((3-(3,4-dihydroxyphenyl)acryloyl)oxy)propanoic acid; MQE6XG29YI; (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoic acid; CHEMBL324842; CHEBI:50371; (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid; (2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]propanoic acid; (2r)-3-(3,4-Dihydroxyphenyl)-2-{[(2e)-3-(3,4-Dihydroxyphenyl)prop-2-Enoyl]oxy}propanoic Acid; Rosemaric acid; (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4-dihydroxyphenyl)lactic acid; (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-propanoic acid; CINNAMIC ACID, 3,4-DIHYDROXY-, 2-ESTER with 3-(3,4-DIHYDROXYPHENYL)LACTIC ACID; 3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester; Benzenepropanoic acid, alpha-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-; UNII-MQE6XG29YI; Rosmarimic acid; CCRIS 9361; RM 21A; HSDB 7688; NPLC 0542; R-(+)-2-(3,4-Dihydroxycinnamoyloxy)-3-(3,4-dihydroxyphenyl)propionic acid; BENZENEPROPANOIC ACID, .ALPHA.-(((2E)-3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPENYL)OXY)-3,4-DIHYDROXY-, (.ALPHA.R)-; Benzenepropanoic acid, .alpha.-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxy-, (.alpha.R)-; MFCD00017740; Rosmarinic acid, 2; ORISTRACT ROA; Rosmarinic acid, 96%; bmse000648; ROSMARINIC ACID [MI]; MLS000697677; ROSMARINIC ACID [HSDB]; ROSMARINIC ACID [INCI]; MEGxp0_000163; RM-21A; SCHEMBL1650675; SCHEMBL2028694; ACon1_001068; CHEBI:92370; cid_5281792; NPLC-0542; DTXSID20896987; ROSMARINIC ACID [USP-RS]; HMS2227A13; HMS3266D13; HMS3411K16; HMS3649C22; HMS3675K16; HMS3885I15; ZINC899870; HY-N0529; BDBM50133496; s3612; ZB1872; 3,4-Dihydroxycinnamic acid 2-ester with 3-(3,4-dihydroxyphenyl)lactic acid; AKOS015892734; CCG-207919; CCG-208268; NCGC00169708-01; AC-33965; AS-35341; Benzenepropanoic acid,a-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxy-,(aR)-; SMR000445579; C01850; EN300-364852; 225R532; A814378; SR-01000946599; Q-100246; SR-01000946599-1; (2R)-O-caffeoyl-3-(3,4-dihydroxyphenyl)lactic acid; Q50380051; F0001-0715; ROSMARINIC ACID (CONSTITUENT OF ROSEMARY) [DSC]; 93B6A3BF-927D-4C59-8A49-29BDBC87C194; Rosmarinic acid, primary pharmaceutical reference standard; ROSMARINIC ACID (CONSTITUENT OF HOLY BASIL LEAF) [DSC]; Rosmarinic acid, European Pharmacopoeia (EP) Reference Standard; Rosmarinic acid, >=98% (HPLC), from Rosemarinus officinalis L.; Rosmarinic acid, United States Pharmacopeia (USP) Reference Standard; (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoicacid; (R)-2-[3-(3,4-Dihydroxyphenyl)acryloyloxy]-3-(3,4-dihydroxyphenyl)propionic acid; (R,E)-3-(3,4-dihydroxyphenyl)-2-((3-(3,4-dihydroxyphenyl)acryloyl)oxy)propanoicacid; (R,E)-3-(3,4-dihydroxyphenyl)-2-(3-(3,4-dihydroxyphenyl)acryloyloxy)propanoic acid; 3-(3,4-DIHYDROXYPHENYL)ACRYLIC ACID-1-CARBOXY-2-(3,4-DIHYDROXYPHENYL)ETHYL ESTER; alpha-(((3,4-Dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxybenzenepropanoic acid; (2R)-3-(3,4-DIHYDROXYPHENYL)-2-{[3-(3,4-DIHYDROXYPHENYL)PROP-2-ENOYL]OXY}PROPANOIC ACID; [R-(+)]-?-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxybenzenepropanoic acid; 162281-84-7; BENZENEPROPANOIC ACID, .ALPHA.-(((2E)-3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPEN-1-YL)OXY)-3,4-DIHYDROXY-, (.ALPHA.R)-; Benzenepropanoic acid, .alpha.-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-, (R-(E))-; Benzenepropanoic acid, alpha-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-, (R-(E))-
|
|
| CAS | 20283-92-5 | |
| PubChem CID | 5281792 | |
| ChEMBL ID | CHEMBL324842 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 360.3 | ALogp: | 2.4 |
| HBD: | 5 | HBA: | 8 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 145.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 26 | QED Weighted: | 0.3 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.005 | MDCK Permeability: | 0.00000694 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.231 | 20% Bioavailability (F20%): | 0.505 |
| 30% Bioavailability (F30%): | 0.994 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.049 | Plasma Protein Binding (PPB): | 97.72% |
| Volume Distribution (VD): | 0.369 | Fu: | 2.04% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.138 | CYP1A2-substrate: | 0.043 |
| CYP2C19-inhibitor: | 0.117 | CYP2C19-substrate: | 0.041 |
| CYP2C9-inhibitor: | 0.554 | CYP2C9-substrate: | 0.798 |
| CYP2D6-inhibitor: | 0.06 | CYP2D6-substrate: | 0.204 |
| CYP3A4-inhibitor: | 0.092 | CYP3A4-substrate: | 0.041 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 15.347 | Half-life (T1/2): | 0.959 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.015 | Human Hepatotoxicity (H-HT): | 0.446 |
| Drug-inuced Liver Injury (DILI): | 0.367 | AMES Toxicity: | 0.035 |
| Rat Oral Acute Toxicity: | 0.451 | Maximum Recommended Daily Dose: | 0.035 |
| Skin Sensitization: | 0.951 | Carcinogencity: | 0.275 |
| Eye Corrosion: | 0.005 | Eye Irritation: | 0.852 |
| Respiratory Toxicity: | 0.052 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002584 |  |
0.456 | D0U3YB |  |
0.489 | ||
| ENC001848 |  |
0.433 | D0KN2M | 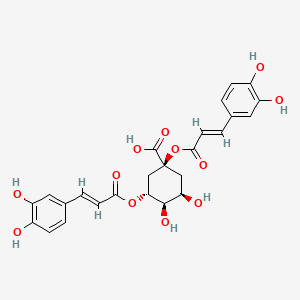 |
0.483 | ||
| ENC001440 | 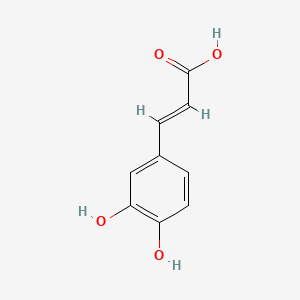 |
0.432 | D0V9EN |  |
0.432 | ||
| ENC001416 | 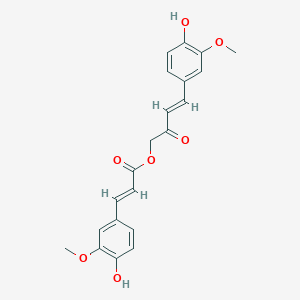 |
0.419 | D08HVR | 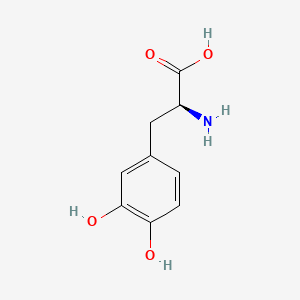 |
0.403 | ||
| ENC002823 |  |
0.409 | D00KRE |  |
0.365 | ||
| ENC001543 |  |
0.408 | D0U0OT |  |
0.354 | ||
| ENC001471 |  |
0.408 | D0BA6T | 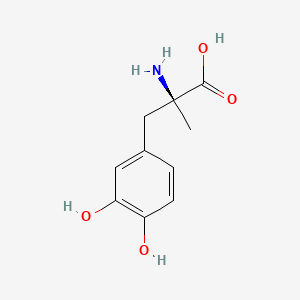 |
0.325 | ||
| ENC001917 | 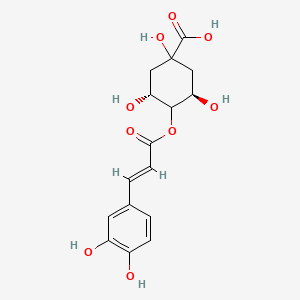 |
0.408 | D02FCQ | 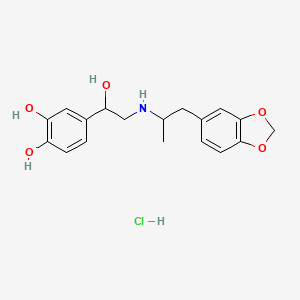 |
0.321 | ||
| ENC000127 | 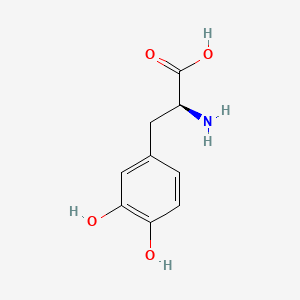 |
0.403 | D0J7RK |  |
0.317 | ||
| ENC002582 |  |
0.395 | D04AIT |  |
0.316 | ||