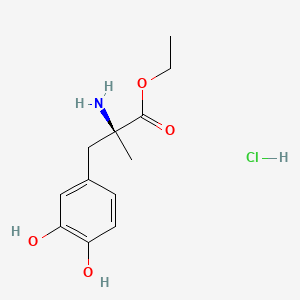
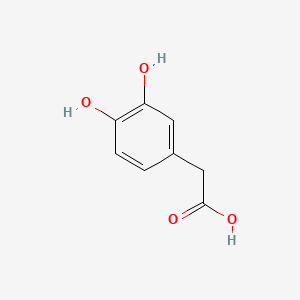
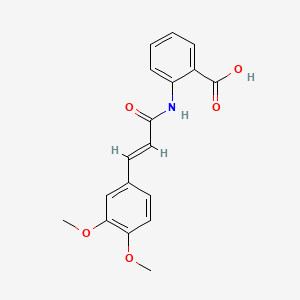
| Synonyms |
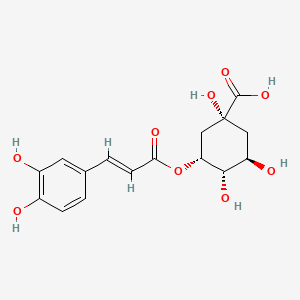
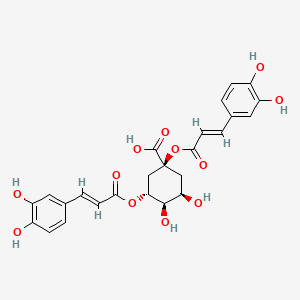
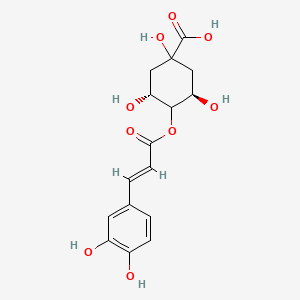
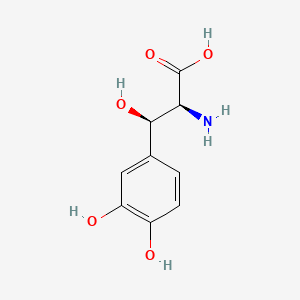
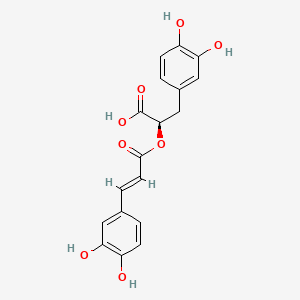
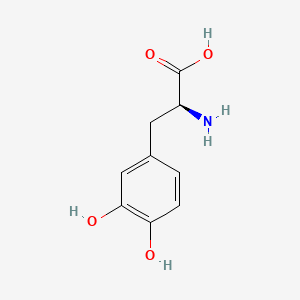
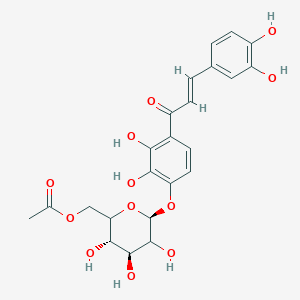
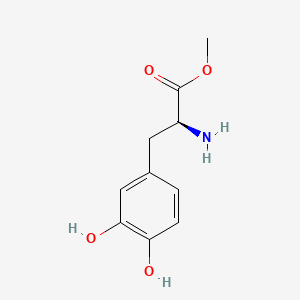
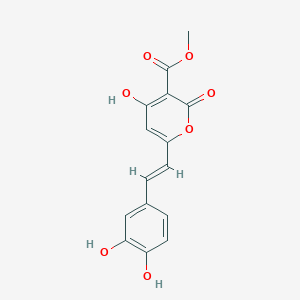
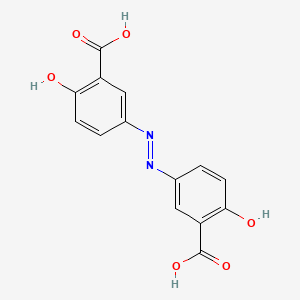
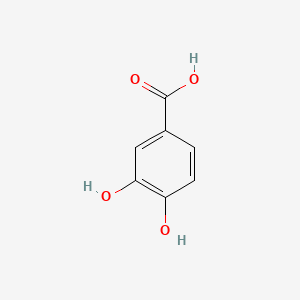
CHLOROGENIC ACID; 327-97-9; 3-O-Caffeoylquinic acid; Heriguard; 3-(3,4-Dihydroxycinnamoyl)quinic acid; 3-Caffeoylquinic acid; Chlorogenate; Hlorogenic acid; CP chlorogenic acid; Chlorogenicacid; NSC-407296; Caffeoyl quinic acid; 5-CQA; (+)-Chlorogenic acid; Chlorogenic acid [MI]; 5-O-(3,4-Dihydroxycinnamoyl)-L-quinic acid; trans-Chlorogenic acid; Caffetannic acid; trans-Caffeic acid 5-o-D-quinate; Chlorogenic acid [WHO-DD]; cis-chlorogenic acid; NSC 70861; NSC-70861; NSC 407296; trans-5-O-Caffeoylquinic acid; 318ADP12RI; CHEMBL284616; (1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylic acid; 202650-88-2; CHEBI:16112; Cyclohexanecarboxylic acid, 3-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,4,5-trihydroxy-, (1S-(1-alpha,3-beta,4-alpha,5-alpha))-; Chlorogenic acid (constituent of st. john's wort) [DSC]; MFCD00003862; 3-O-(3,4-Dihydroxycinnamoyl)-D-quinic acid; (1S,3R,4R,5R)-3-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexane-1-carboxylic acid; Cyclohexanecarboxylic acid, 3-(((2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-1,4,5-trihydroxy-, (1S,3R,4R,5R)-; Cyclohexanecarboxylic acid, 3-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,4,5-trihydroxy-, (1S,3R,4R,5R)-; Cyclohexanecarboxylic acid, 3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxy-, (1S,3R,4R,5R)-; caffeoylquinic acid; 1,3,4,5-tetrahydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate); 3-O-caffeoyl-D-quinic acid; 1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate; 3-Caffeoylquinate; 5-Caffeoylquinic acid; 3-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]oxy] 1,4,5-trihydroxycyclohexanecarboxylic acid; 5-caffeoyl quinic acid; (1S,3R,4R,5R)-3-(((3-(3,4-dihydroxyphenyl)acryloyl)oxy)-1,4,5-trihydroxycyclohexanecarboxylic acid; [1S-(1alpha,3beta,4alpha,5alpha)]-3-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxycyclohexanecarboxylic acid; [1S-(1alpha,3beta,4alpha,5alpha)]3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxycyclohexanecarboxylic acid; cyclohexanecarboxylic acid, 3-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxy-, (1S,3R,4R,5R)-; Cyclohexanecarboxylic acid, 3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxy-, [1S-(1alpha,3beta,4alpha,5alpha)]-; CCRIS 1400; EINECS 206-325-6; 3-trans-Caffeoylquinic acid; UNII-318ADP12RI; Hlorogenate; Helianthic acid; Igasuric acid; NSC70861; Chlorogenic-acid; NSC407296; Caffeylquinic acid; 3-(3,4-Dihydroxycinnamoyl)quinate; Prestwick_112; (E)-chlorogenic acid; Chlorogenic acid, Chiral; Chlorogenic acid (8CI); Prestwick2_000427; Prestwick3_000427; Spectrum5_000733; bmse000387; Quinic acid, 5-caffeoyl-; SCHEMBL19466; BSPBio_000414; BSPBio_003353; SPECTRUM210800; MLS002153805; BIDD:ER0453; BPBio1_000456; Quinic acid, 3-caffeoyl-, E-; ACon1_000581; CHEBI:95271; CHLOROGENIC ACID [USP-RS]; BDBM513080; DTXSID101318952; HMS1569E16; HMS1923C11; HMS2096E16; HMS2235F03; HMS3649E06; ACT03375; ALBB-030169; CGA; HY-N0055; ZINC2138728; BDBM50327036; CCG-38471; s2280; AKOS015955866; AC-6032; Chlorogenic acid, >=95% (titration); CS-3766; DB12029; SDCCGMLS-0066467.P001; NCGC00168941-01; NCGC00168941-02; NCGC00168941-03; (1S,3R,4R,5R)-3-(((E)-3-(3,4-dihydroxyphenyl)acryloyl)oxy)-1,4,5-trihydroxycyclohexane-1-carboxylic acid; (1S,3R,4R,5R)-3-[(E)-3-(3,4-DIHYDROXY-PHENYL)-ACRYLOYLOXY]-1,4,5-TRIHYDROXY-CYCLOHEXANECARBOXYLIC ACID; (1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylicacid; AS-12284; SMR000857273; Chlorogenic acid 1000 microg/mL in Acetone; Chlorogenic acid 10 microg/mL in Acetonitrile; C00852; F16266; 327C979; Q421964; SR-01000841185; SR-01000946600; D54CAE3D-CDDA-455D-A28E-77FC9EFE4A43; SR-01000841185-4; SR-01000946600-1; BRD-K47114202-001-06-2; 32CF6D13-8F08-485F-B79E-F8A6AC318E07; Chlorogenic acid, primary pharmaceutical reference standard; Chlorogenic acid, European Pharmacopoeia (EP) Reference Standard; Chlorogenic acid, United States Pharmacopeia (USP) Reference Standard; 3-[(E)-3-(3,4-Dihydroxy-phenyl)-acryloyloxy]-1,4,5-trihydroxy-cyclohexanecarboxylic acid; 3-[3-(3,4-Dihydroxy-phenyl)-acryloyloxy]-1,4,5-trihydroxy-cyclohexanecarboxylic acid; (1R,3S,4S,5S)-3-[(E)-3-(3,4-Dihydroxy-phenyl)-acryloyloxy]-1,4,5-trihydroxy-cyclohexanecarboxylic acid; (1S,3R,4R,5R)-3-(((3-(3,4-dihydroxyphenyl)acryloyl)oxy)-1,4,5-trihydroxycyclohexanecarboxylicacid; (1S,3R,4R,5R)-3-(((E)-3-(3,4-dihydroxyphenyl)acryloyl)oxy)-1,4,5-trihydroxycyclohexanecarboxylic acid; (1S,3R,4R,5R)-3-((E)-3-(3,4-dihydroxyphenyl)acryloyloxy)-1,4,5-trihydroxycyclohexanecarboxylic acid; (1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxy-cyclohexanecarboxylic acid; (1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxycyclohexanecarboxylic acid; (1S,3R,4R,5R,E)-3-(3-(3,4-dihydroxyphenyl)acryloyloxy)-1,4,5-trihydroxycyclohexanecarboxylic acid; CYCLOHEXANECARBOXYLIC ACID, 3-((3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPENYL)OXY)-1,4,5-TRIHYDROXY-, (1S-(1-.ALPHA.,3-.BETA.,4-.ALPHA.,5-.ALPHA.))-; Cyclohexanecarboxylic acid,3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxy-,(1S,3R,4R,5R)-; edit(1S,3R,4R,5R)-3-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexane-1-carboxylic acid
|