NPs Basic Information
|
Name |
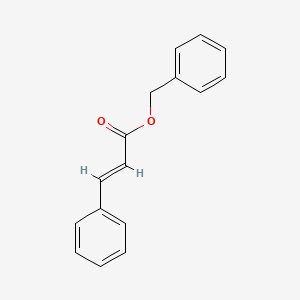
Benzyl cinnamate
|
| Molecular Formula | C16H14O2 | |
| IUPAC Name* |
benzyl (E)-3-phenylprop-2-enoate
|
|
| SMILES |
C1=CC=C(C=C1)COC(=O)/C=C/C2=CC=CC=C2
|
|
| InChI |
InChI=1S/C16H14O2/c17-16(12-11-14-7-3-1-4-8-14)18-13-15-9-5-2-6-10-15/h1-12H,13H2/b12-11+
|
|
| InChIKey |
NGHOLYJTSCBCGC-VAWYXSNFSA-N
|
|
| Synonyms |
BENZYL CINNAMATE; 103-41-3; Cinnamein; Benzyl 3-phenylpropenoate; Cinnamic acid benzyl ester; Cinnamic acid, benzyl ester; Benzylcinnamate; Benzyl alcohol, cinnamic ester; FEMA No. 2142; trans-Cinnamic acid benzyl ester; benzyl (E)-3-phenylprop-2-enoate; NSC 11780; Benzylester kyseliny skoricove; Benzyltrans Cinnamate; Benzyl (E)-Cinnamate; Benzyl alcohol, cinnamate; 3-Phenyl-2-propenoic acid phenylmethyl ester; Benzyl .gamma.-phenylacrylate; Benzyl 3-phenyl-2-propenoate; 2-Propenoic acid, 3-phenyl-, phenylmethyl ester; CHEMBL361197; V67O3RO97U; benzyl (2E)-3-phenylprop-2-enoate; 78277-23-3; 3-Phenyl-2-propenoic acid benzyl ester; Benzylcinnamoate; Benzyl-3-phenylpropenoate; MFCD00004789; Benzyl gamma-phenylacrylate; HSDB 359; Phenylmethyl 3-phenyl-2-propenoate; EINECS 203-109-3; Benzylester kyseliny skoricove [Czech]; UNII-V67O3RO97U; AI3-01268; NSC-11780; NSC-44403; NCGC00166124-01; Cinnamic acid benzyl; Benzyl benzeneacrylate; benzyl trans-cinnamate; Benzyl cinnamate, 99%; benzyl cinnamate (trans); Benzyl cinnamate, >=98%; SCHEMBL43212; BENZYL CINNAMATE [MI]; benzyl (2E)-3-phenylacrylate; BENZYL CINNAMATE [FCC]; BENZYL CINNAMATE [FHFI]; BENZYL CINNAMATE [HSDB]; BENZYL CINNAMATE [INCI]; 2-Propenoic acid, 3-phenyl-, phenylmethyl ester, (E)-; BENZYL CINNAMATE, (E)-; BENZYL CINNAMATE [MART.]; DTXSID00880905; BENZYL CINNAMATE [WHO-DD]; CHEBI:146174; 8014-16-2; HY-N7090; 3-Phenyl-acrylic acid, benzyl ester; BDBM50149609; s5167; STL282668; ZINC12358883; AKOS002944462; CCG-266864; CS-W010306; (E)-3-Phenyl-acrylic acid benzyl ester; NCGC00166124-02; trans-3-Phenyl-acrylic acid benzyl ester; AS-14650; 3-phenyl-2-propenoic acid, phenylmethyl ester; Benzyl cinnamate, analytical reference material; A800730; SR-01000944730; benzyl (E)-3-phenylprop-2-enoate;Benzyl cinnamate; J-000961; Q9197460; SR-01000944730-1; Z19782660; 2-Propenoic acid, 3-phenyl-, phenylmethyl ester, (2E)-
|
|
| CAS | 103-41-3 | |
| PubChem CID | 5273469 | |
| ChEMBL ID | CHEMBL361197 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 238.28 | ALogp: | 3.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 18 | QED Weighted: | 0.589 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.616 | MDCK Permeability: | 0.00002230 |
| Pgp-inhibitor: | 0.031 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.694 |
| 30% Bioavailability (F30%): | 0.019 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.797 | Plasma Protein Binding (PPB): | 96.45% |
| Volume Distribution (VD): | 0.652 | Fu: | 3.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.992 | CYP1A2-substrate: | 0.128 |
| CYP2C19-inhibitor: | 0.968 | CYP2C19-substrate: | 0.065 |
| CYP2C9-inhibitor: | 0.876 | CYP2C9-substrate: | 0.859 |
| CYP2D6-inhibitor: | 0.379 | CYP2D6-substrate: | 0.356 |
| CYP3A4-inhibitor: | 0.266 | CYP3A4-substrate: | 0.277 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.227 | Half-life (T1/2): | 0.571 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.071 | Human Hepatotoxicity (H-HT): | 0.027 |
| Drug-inuced Liver Injury (DILI): | 0.8 | AMES Toxicity: | 0.527 |
| Rat Oral Acute Toxicity: | 0.011 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.939 | Carcinogencity: | 0.663 |
| Eye Corrosion: | 0.384 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.044 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001737 |  |
0.817 | D0G1VX |  |
0.667 | ||
| ENC000077 |  |
0.667 | D01ZJK |  |
0.448 | ||
| ENC001428 |  |
0.569 | D0T5UL | 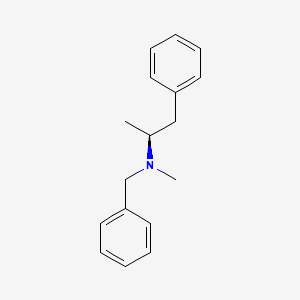 |
0.438 | ||
| ENC000093 |  |
0.492 | D04DXN | 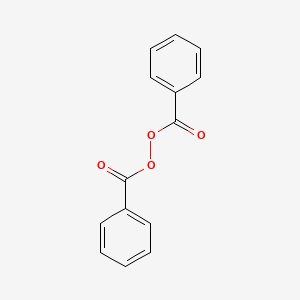 |
0.438 | ||
| ENC003616 | 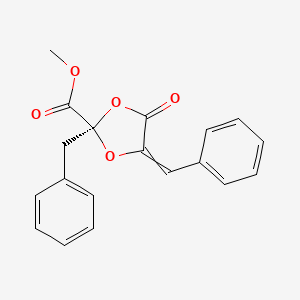 |
0.488 | D07HQC | 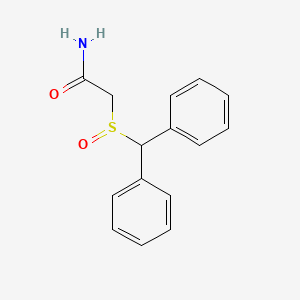 |
0.427 | ||
| ENC001449 | 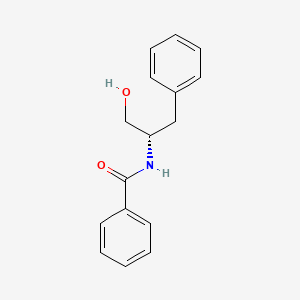 |
0.479 | D0J5RN | 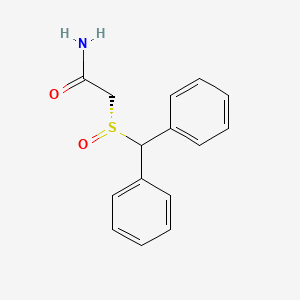 |
0.427 | ||
| ENC000302 | 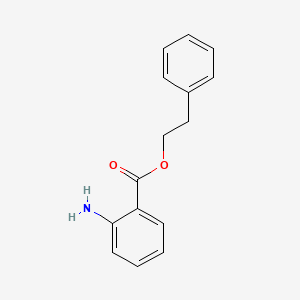 |
0.479 | D00HPK | 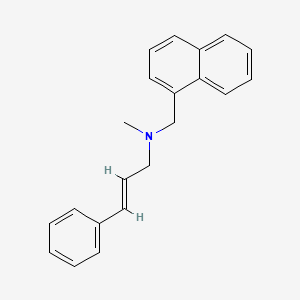 |
0.422 | ||
| ENC000295 | 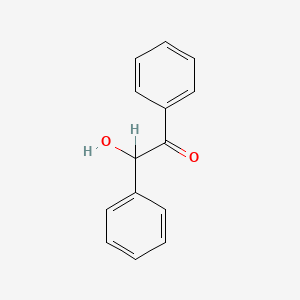 |
0.456 | D0D4PB | 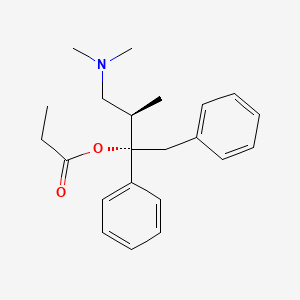 |
0.414 | ||
| ENC000308 | 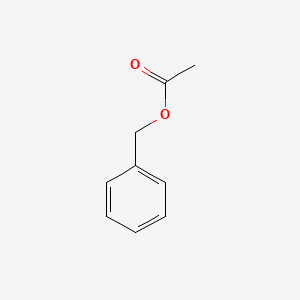 |
0.448 | D03HCZ | 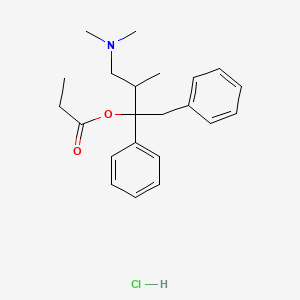 |
0.409 | ||
| ENC001091 | 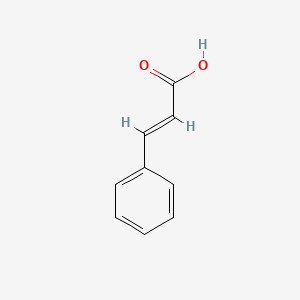 |
0.448 | D0X2DK | 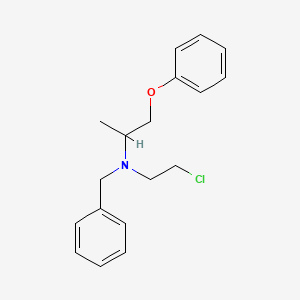 |
0.407 | ||