NPs Basic Information
|
Name |
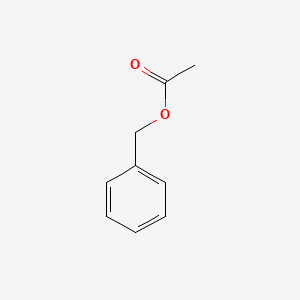
Benzyl acetate
|
| Molecular Formula | C9H10O2 | |
| IUPAC Name* |
benzyl acetate
|
|
| SMILES |
CC(=O)OCC1=CC=CC=C1
|
|
| InChI |
InChI=1S/C9H10O2/c1-8(10)11-7-9-5-3-2-4-6-9/h2-6H,7H2,1H3
|
|
| InChIKey |
QUKGYYKBILRGFE-UHFFFAOYSA-N
|
|
| Synonyms |
BENZYL ACETATE; 140-11-4; Benzyl ethanoate; Phenylmethyl acetate; Acetic acid benzyl ester; Acetic acid, phenylmethyl ester; Acetic acid, benzyl ester; Phenylmethyl ethanoate; alpha-Acetoxytoluene; (Acetoxymethyl)benzene; NCI-C06508; Benzylester kyseliny octove; FEMA No. 2135; Plastolin I; NSC 4550; .alpha.-Acetoxytoluene; Benzyl ester of acetic acid; 0ECG3V79ZJ; CHEBI:52051; NSC-4550; Benzyl acetate + glycine combination; DSSTox_CID_151; DSSTox_RID_75403; DSSTox_GSID_20151; Caswell No. 081EA; Benzyl acetate (natural); CAS-140-11-4; Acetic acid phenylmethyl ester; CCRIS 1423; HSDB 2851; Benzylester kyseliny octove [Czech]; ACETATO DE BENCILO; EINECS 205-399-7; UNII-0ECG3V79ZJ; Benzyl acetate, primary pharmaceutical reference standard; AI3-01996; FEMA 2135; Acetic acid benzyl; J0Z; MFCD00008712; nchem.167-comp5; Benzyl Acetate Natural; Acetic acid-benzyl ester; Benzyl acetate, >=99%; EC 205-399-7; BENZYL ACETATE [MI]; WLN: 1VO1R; SCHEMBL43745; BENZYL ACETATE [FCC]; Acetic acid,phenylmethyl ester; BENZYL ACETATE [FHFI]; BENZYL ACETATE [HSDB]; BENZYL ACETATE [IARC]; BENZYL ACETATE [INCI]; BENZYL ACETATE [VANDF]; CHEMBL1233714; DTXSID0020151; AMY3828; NSC4550; ZINC157134; Benzyl acetate, analytical standard; HY-N7124; Tox21_201826; Tox21_302841; s5576; STL283809; Benzyl acetate, >=99%, FCC, FG; AKOS015841099; CCG-266204; CS-W018145; NCGC00090779-01; NCGC00090779-02; NCGC00090779-03; NCGC00256379-01; NCGC00259375-01; LS-13613; A0022; Benzyl acetate, natural, >=99%, FCC, FG; E1501; FT-0621741; Benzyl acetate, Selectophore(TM), >=99.5%; EN300-1267317; Q424223; J-007357; W-200649; Z19628364
|
|
| CAS | 140-11-4 | |
| PubChem CID | 8785 | |
| ChEMBL ID | CHEMBL1233714 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.17 | ALogp: | 2.0 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.605 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.262 | MDCK Permeability: | 0.00004430 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.022 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.989 | Plasma Protein Binding (PPB): | 43.34% |
| Volume Distribution (VD): | 1.257 | Fu: | 54.77% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.947 | CYP1A2-substrate: | 0.107 |
| CYP2C19-inhibitor: | 0.627 | CYP2C19-substrate: | 0.267 |
| CYP2C9-inhibitor: | 0.08 | CYP2C9-substrate: | 0.121 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.172 |
| CYP3A4-inhibitor: | 0.014 | CYP3A4-substrate: | 0.362 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.409 | Half-life (T1/2): | 0.863 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.064 | Human Hepatotoxicity (H-HT): | 0.066 |
| Drug-inuced Liver Injury (DILI): | 0.791 | AMES Toxicity: | 0.29 |
| Rat Oral Acute Toxicity: | 0.028 | Maximum Recommended Daily Dose: | 0.012 |
| Skin Sensitization: | 0.372 | Carcinogencity: | 0.423 |
| Eye Corrosion: | 0.625 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.03 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000216 | 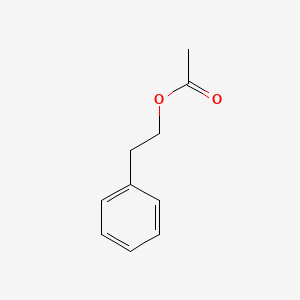 |
0.711 | D0G1VX |  |
0.500 | ||
| ENC000596 | 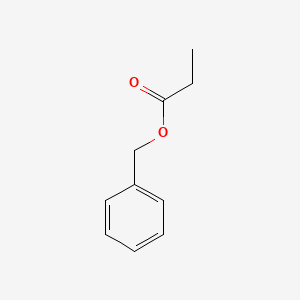 |
0.711 | D05OIS |  |
0.500 | ||
| ENC000214 | 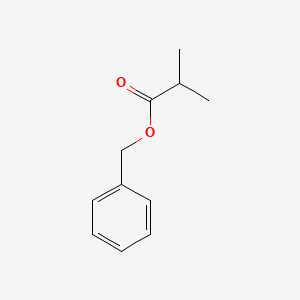 |
0.675 | D0R1CR |  |
0.488 | ||
| ENC000215 | 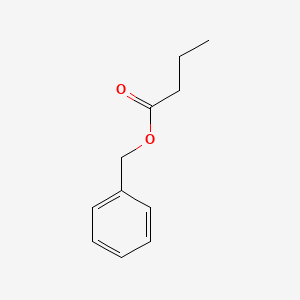 |
0.659 | D0T3LF | 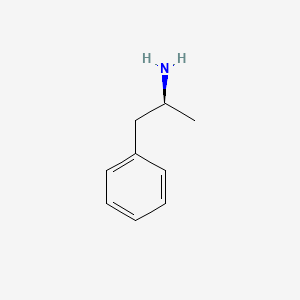 |
0.475 | ||
| ENC000598 | 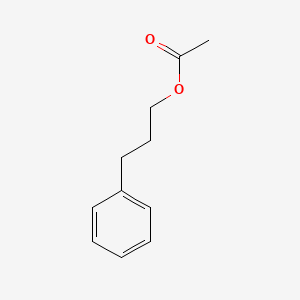 |
0.659 | D05BMG |  |
0.475 | ||
| ENC000218 | 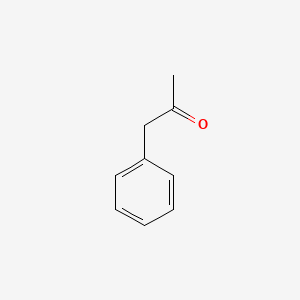 |
0.639 | D0P2GK | 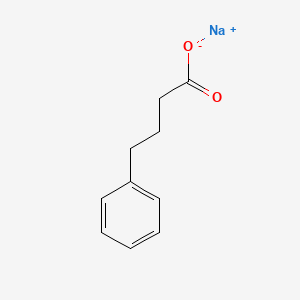 |
0.467 | ||
| ENC000208 | 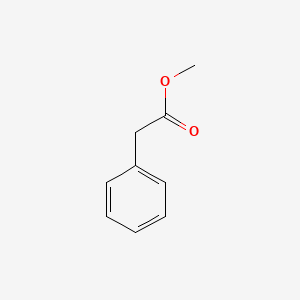 |
0.590 | D07ONP | 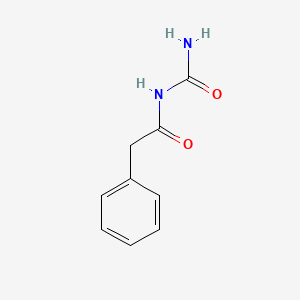 |
0.457 | ||
| ENC001819 | 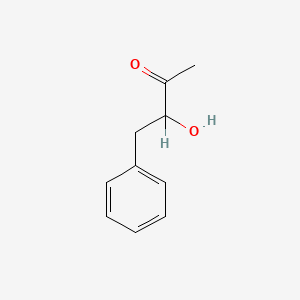 |
0.561 | D0U0RZ | 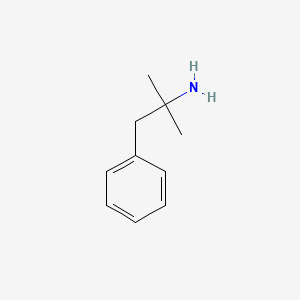 |
0.452 | ||
| ENC000054 | 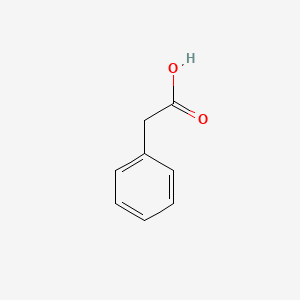 |
0.553 | D00DZN |  |
0.447 | ||
| ENC005854 |  |
0.553 | D0P6UB | 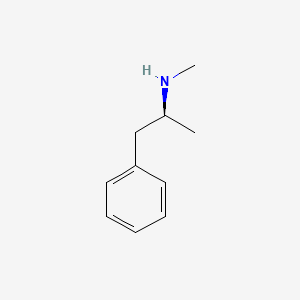 |
0.442 | ||