NPs Basic Information

|
Name |
Benzophenone
|
| Molecular Formula | C13H10O | |
| IUPAC Name* |
diphenylmethanone
|
|
| SMILES |
C1=CC=C(C=C1)C(=O)C2=CC=CC=C2
|
|
| InChI |
InChI=1S/C13H10O/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
|
|
| InChIKey |
RWCCWEUUXYIKHB-UHFFFAOYSA-N
|
|
| Synonyms |
BENZOPHENONE; 119-61-9; diphenylmethanone; Diphenyl ketone; Benzoylbenzene; Methanone, diphenyl-; Phenyl ketone; Ketone, diphenyl; alpha-Oxoditane; Benzene, benzoyl-; alpha-Oxodiphenylmethane; Diphenylketone; diphenyl-methanone; Kayacure bp; .alpha.-Oxoditane; Adjutan 6016; FEMA No. 2134; .alpha.-Oxodiphenylmethane; Diphenyl-methanon; 1dzp; NSC 8077; MFCD00003076; CHEMBL90039; 701M4TTV9O; DTXSID0021961; CHEBI:41308; NSC-8077; NCGC00090787-05; DSSTox_CID_1961; DSSTox_RID_76429; DSSTox_GSID_21961; Diphenylmethanone (Benzophenone); Caswell No. 081G; CAS-119-61-9; CCRIS 629; HSDB 6809; WLN: RVR; EINECS 204-337-6; EPA Pesticide Chemical Code 000315; BENZOPHENONE (8CI); phenylketone; UNII-701M4TTV9O; Benzopheneone; Benzophenon; benzophenone-; benzoyl-benzene; a-Oxoditane; AI3-00754; meta-benzophenone; alpha -oxoditane; FEMA 2134; Benzophenone Flakes; di(phenyl)methanone; a-Oxodiphenylmethane; METHANONE, DIPHENYL- (9CI); Ph2CO; SPEEDCURE BP; DAROCUR BP; Diphenylmethanone, 9CI; alpha -oxodiphenylmethane; Dimenhydrinate Impurity J; BENZOPHENONE [MI]; BENZOPHENONE [FCC]; UPCMLD-DP071; BENZOPHENONE [FHFI]; BENZOPHENONE [HSDB]; BENZOPHENONE [IARC]; BENZOPHENONE [INCI]; EC 204-337-6; BIDD:PXR0008; SCHEMBL17745; MLS001055400; ADK STAB 1413; BENZOPHENONE [USP-RS]; BENZOPHENONE [WHO-DD]; BIDD:ER0022; Benzophenone (diphenyl-ketone); Benzophenone (Diphenylmethanone); UPCMLD-DP071:001; BDBM22726; Benzophenone, analytical standard; DIPHENHYDRAMINE IMPURITY E; AMY7704; NSC8077; HMS2268A24; ZINC968233; BENZOPHENONE [USP IMPURITY]; Benzophenone, >=99%, FCC, FG; HY-Y0546; Tox21_113465; Tox21_202425; Tox21_300058; Benzophenone, ReagentPlus(R), 99%; s4438; STL363250; Benzophenone, for synthesis, 98.0%; AKOS000119029; Tox21_113465_1; BENZOPHENONE (DIPHENYL-KETONE); DB01878; Benzophenone, purum, >=99.0% (GC); Benzophenone, ReagentPlus(R), >=99%; NCGC00090787-01; NCGC00090787-03; NCGC00090787-04; NCGC00090787-06; NCGC00090787-07; NCGC00090787-08; NCGC00254183-01; NCGC00259974-01; BP-21212; SMR000112143; PHENYTOIN IMPURITY A [EP IMPURITY]; Benzophenone, SAJ first grade, >=99.0%; DB-061602; B0083; CS-0015323; FT-0622720; FT-0693251; Benzophenone, purified by sublimation, >=99%; Benzophenone, Vetec(TM) reagent grade, 98%; EN300-19181; C06354; D72506; DIMENHYDRINATE IMPURITY J [EP IMPURITY]; PHENYTOIN SODIUM IMPURITY A [EP IMPURITY]; Q409482; Melting point standard 47-49C, analytical standard; Q-200691; PHENYTOIN IMPURITY BENZOPHENONE [USP IMPURITY]; F0001-0309; Z104473064; Benzophenone, European Pharmacopoeia (EP) Reference Standard; DIPHENHYDRAMINE HYDROCHLORIDE IMPURITY E [EP IMPURITY]; Mettler-Toledo Calibration substance ME 18870, Benzophenone; PHENYTOIN SODIUM IMPURITY BENZOPHENONE [USP IMPURITY]; Benzophenone, United States Pharmacopeia (USP) Reference Standard; HYDROCODONE HYDROGEN TARTRATE 2.5-HYDRATE IMPURITY H [EP IMPURITY]; Benzophenone, Pharmaceutical Secondary Standard; Certified Reference Material; Mettler-Toledo Calibration substance ME 18870, Benzophenone, for the calibration of the thermosystem 900, traceable to primary standards (LGC)
|
|
| CAS | 119-61-9 | |
| PubChem CID | 3102 | |
| ChEMBL ID | CHEMBL90039 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.22 | ALogp: | 3.4 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 14 | QED Weighted: | 0.647 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.334 | MDCK Permeability: | 0.00001760 |
| Pgp-inhibitor: | 0.052 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.235 |
| 30% Bioavailability (F30%): | 0.17 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.233 | Plasma Protein Binding (PPB): | 98.05% |
| Volume Distribution (VD): | 0.658 | Fu: | 1.67% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.988 | CYP1A2-substrate: | 0.322 |
| CYP2C19-inhibitor: | 0.853 | CYP2C19-substrate: | 0.113 |
| CYP2C9-inhibitor: | 0.693 | CYP2C9-substrate: | 0.267 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.086 |
| CYP3A4-inhibitor: | 0.023 | CYP3A4-substrate: | 0.243 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.416 | Half-life (T1/2): | 0.665 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.098 | Human Hepatotoxicity (H-HT): | 0.043 |
| Drug-inuced Liver Injury (DILI): | 0.957 | AMES Toxicity: | 0.042 |
| Rat Oral Acute Toxicity: | 0.045 | Maximum Recommended Daily Dose: | 0.029 |
| Skin Sensitization: | 0.254 | Carcinogencity: | 0.739 |
| Eye Corrosion: | 0.006 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.086 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000295 | 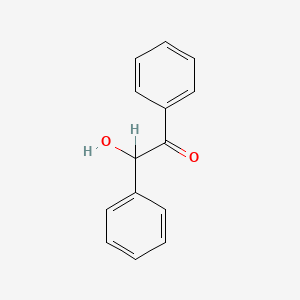 |
0.642 | D04DXN | 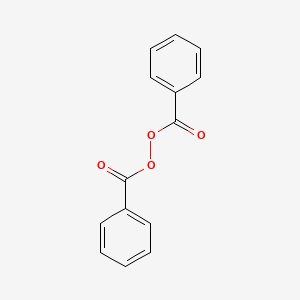 |
0.661 | ||
| ENC000077 | 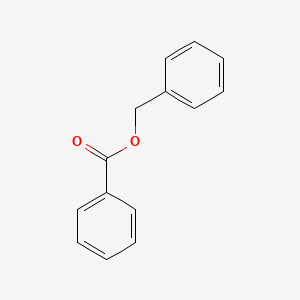 |
0.630 | D0G1VX |  |
0.630 | ||
| ENC001449 | 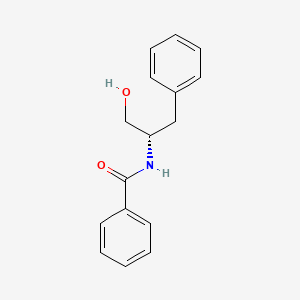 |
0.548 | D0W9WF | 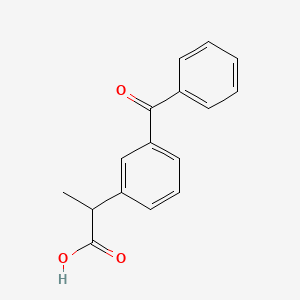 |
0.541 | ||
| ENC000461 | 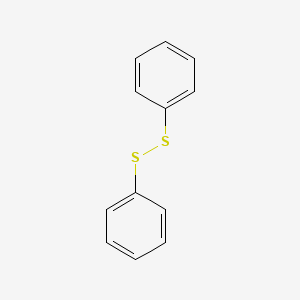 |
0.509 | D0E8LT |  |
0.486 | ||
| ENC001523 | 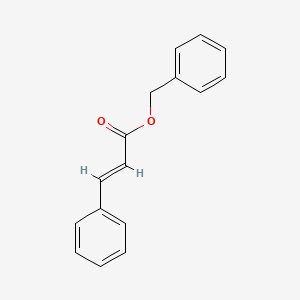 |
0.492 | D07HQC | 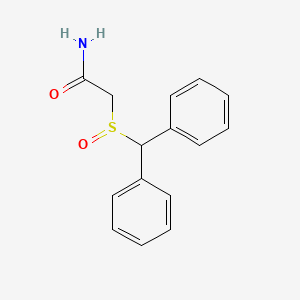 |
0.484 | ||
| ENC001737 | 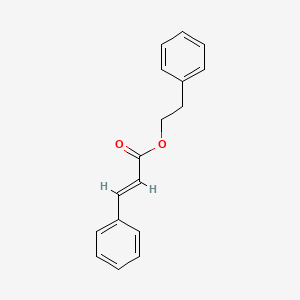 |
0.470 | D0J5RN | 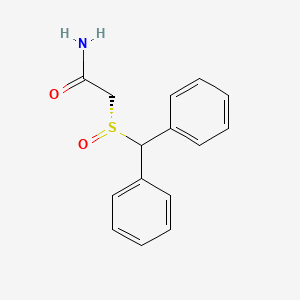 |
0.484 | ||
| ENC001428 | 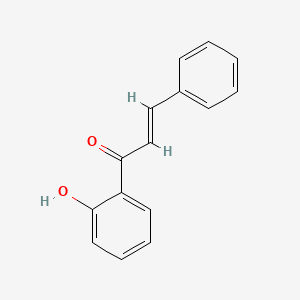 |
0.452 | D0OK5Q | 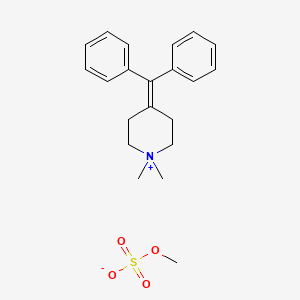 |
0.456 | ||
| ENC000047 | 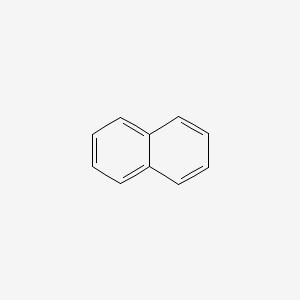 |
0.449 | D02IHW | 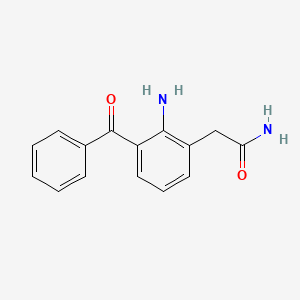 |
0.446 | ||
| ENC001050 |  |
0.446 | D0X9RY |  |
0.435 | ||
| ENC000076 | 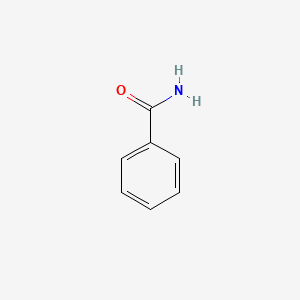 |
0.435 | D0E4DW | 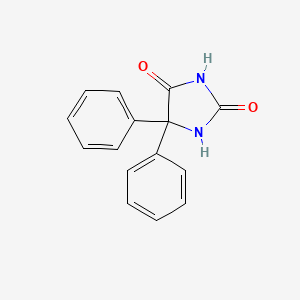 |
0.433 | ||