NPs Basic Information
|
Name |
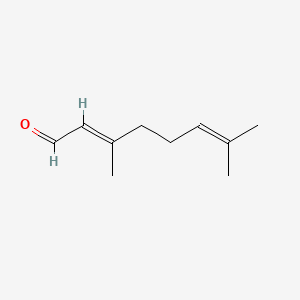
Citral
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
(2E)-3,7-dimethylocta-2,6-dienal
|
|
| SMILES |
CC(=CCC/C(=C/C=O)/C)C
|
|
| InChI |
InChI=1S/C10H16O/c1-9(2)5-4-6-10(3)7-8-11/h5,7-8H,4,6H2,1-3H3/b10-7+
|
|
| InChIKey |
WTEVQBCEXWBHNA-JXMROGBWSA-N
|
|
| Synonyms |
Citral; GERANIAL; 5392-40-5; trans-Citral; (2E)-3,7-dimethylocta-2,6-dienal; 141-27-5; 3,7-dimethylocta-2,6-dienal; Citral a; geranialdehyde; (E)-Citral; Geranaldehyde; 2,6-Octadienal, 3,7-dimethyl-; alpha-Citral; (E)-Geranial; geranal; Genanial; beta-Geranial; (E)-3,7-Dimethylocta-2,6-dienal; 3,7-Dimethyl-2,6-octadienal; (E)-Neral; 2,6-Octadienal, 3,7-dimethyl-, (2E)-; 147060-73-9; Lemsyn GB; trans-3,7-Dimethyl-2,6-octadienal; Natural Citral; NCI-C56348; 2,6-Octadienal, 3,7-dimethyl-, (E)-; Citral-A; (E)-3,7-Dimethyl-2,6-octadienal; 3,7-Dimethyl-trans-2,6-octadienal; FEMA No. 2303; cis/trans-3,7-Dimethyl-2,6-octadienal; citral-b; Lemarome n; CHEBI:16980; NSC6170; 758ZMW724E; trans-Citral = trans-3,7-Dimethyl-octa-2,6-dien-1-al; cis-3,7-Dimethyl-2,6-octadienal; NSC 6170; Citral alpha; Citral, analytical standard; Citral (natural); CITRAL NATURAL; Z-Citral; Caswell No. 221B; FEMA Number 2303; CCRIS 1043; HSDB 993; CITRAL SINTETICO; 2,6-Dimethyloctadien-2,6-al-8; 3,7-Dimethyl-1,2,6-octadienal; EINECS 205-476-5; EINECS 226-394-6; UNII-T7EU0O9VPP; EPA Pesticide Chemical Code 040510; BRN 1721871; BRN 1721873; UNII-758ZMW724E; AI3-01011; AI3-28519; alpha -Citral; Citral N; .alpha.-Citral; 3,6-octadienal; (2e)-geranial; LEMAROME; 3,2,6-octadienal; (2E)-3,7-Dimethyl-2,6-octadienal; CITRAL GERANIAL; Citral, cis + trans; Citral, 95%; T7EU0O9VPP; EC 205-476-5; EC 226-394-6; Citral Ex Litsea(Citral ); SCHEMBL23073; CITRAL GERANIAL [MI]; 3-01-00-03053 (Beilstein Handbook Reference); 4-01-00-03569 (Beilstein Handbook Reference); GTPL6327; CHEMBL1080997; 3,7- dimethylocta-2,6-dienal; DTXSID20881217; CHEBI:137934; WLN: VH1UY1&3Y1&U1; HY-N7083; NSC-6170; ZINC1529208; 3,7-dimethyl-(e)-2,6-octadienal; BBL011666; MFCD00006997; STK802499; 3,7-dimethyl-(2e)-2,6-octadienal; trans-3,7-dimethyl-octa-2,6-dienal; AKOS000119519; CCG-266236; Citral, natural, >=96%, FCC, FG; CS-W010948; LMPR0102010003; (2E)-3,7-dimethyl-octa-2,6-dienal; Citral 1000 microg/mL in Acetonitrile; Citral, Vetec(TM) reagent grade, 94%; NCGC00091550-01; NCGC00091550-02; NCGC00091550-03; NCGC00091550-04; AS-35309; (2E)-3,7-dimethyl-2,6-octadien-1-al; 2,6- OCTADIENAL, 3,7-DIMETHYL-; S5138; 3,7-DIMETHYL-2,6-OCTADIENAL(TRANS); EN300-20399; C01499; A829835; Q410888; Q-200867; F0001-1403; Z104478042; 2,6-Octadienal,3,7-dimethyl-,reaction products with et alc.; GRQ
|
|
| CAS | 5392-40-5 | |
| PubChem CID | 638011 | |
| ChEMBL ID | CHEMBL1080997 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 3.0 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.341 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.309 | MDCK Permeability: | 0.00002880 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.114 |
| 30% Bioavailability (F30%): | 0.029 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.918 | Plasma Protein Binding (PPB): | 92.92% |
| Volume Distribution (VD): | 2.827 | Fu: | 10.91% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.781 | CYP1A2-substrate: | 0.639 |
| CYP2C19-inhibitor: | 0.346 | CYP2C19-substrate: | 0.867 |
| CYP2C9-inhibitor: | 0.067 | CYP2C9-substrate: | 0.902 |
| CYP2D6-inhibitor: | 0.044 | CYP2D6-substrate: | 0.59 |
| CYP3A4-inhibitor: | 0.02 | CYP3A4-substrate: | 0.226 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.513 | Half-life (T1/2): | 0.618 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.535 |
| Drug-inuced Liver Injury (DILI): | 0.072 | AMES Toxicity: | 0.427 |
| Rat Oral Acute Toxicity: | 0.036 | Maximum Recommended Daily Dose: | 0.058 |
| Skin Sensitization: | 0.928 | Carcinogencity: | 0.88 |
| Eye Corrosion: | 0.904 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.931 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001434 | 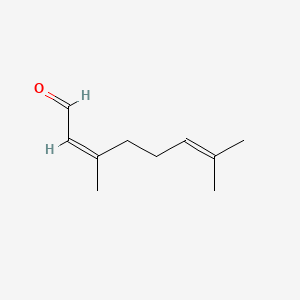 |
1.000 | D0M1PQ | 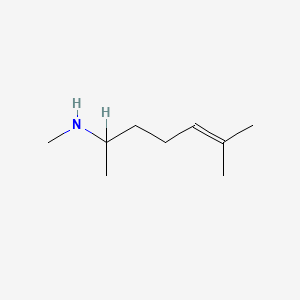 |
0.341 | ||
| ENC001717 | 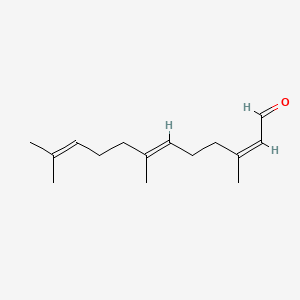 |
0.674 | D05XQE | 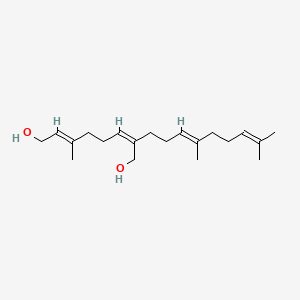 |
0.328 | ||
| ENC001718 | 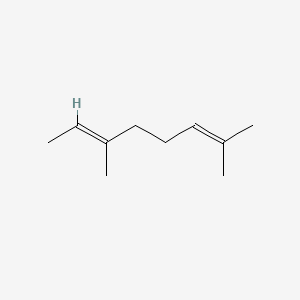 |
0.618 | D09XWD | 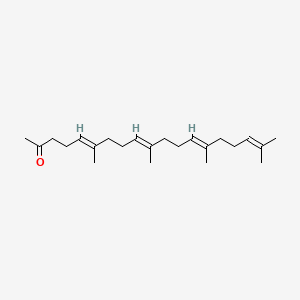 |
0.324 | ||
| ENC001649 | 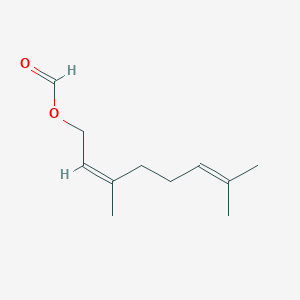 |
0.600 | D03VFL | 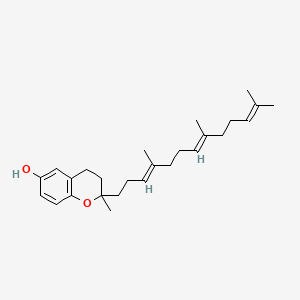 |
0.259 | ||
| ENC001719 | 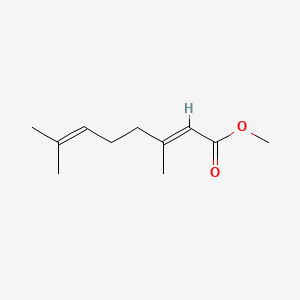 |
0.537 | D0Q6DX | 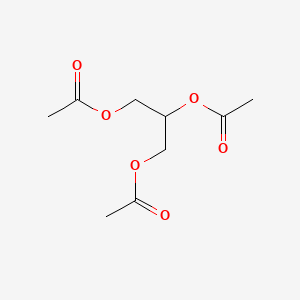 |
0.193 | ||
| ENC001467 | 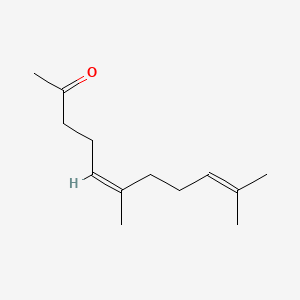 |
0.500 | D0F1GS |  |
0.179 | ||
| ENC001664 |  |
0.468 | D0Z4NI |  |
0.179 | ||
| ENC000314 |  |
0.449 | D0S7WX | 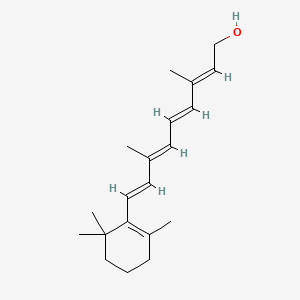 |
0.178 | ||
| ENC001096 | 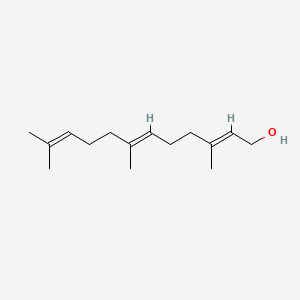 |
0.440 | D03ZFG | 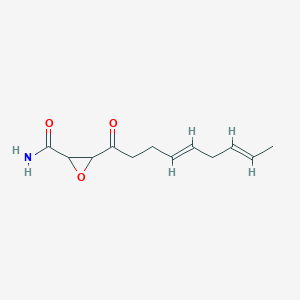 |
0.177 | ||
| ENC000230 | 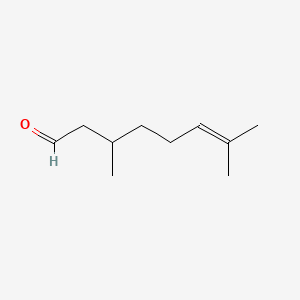 |
0.415 | D0X7XG |  |
0.174 | ||