NPs Basic Information
|
Name |
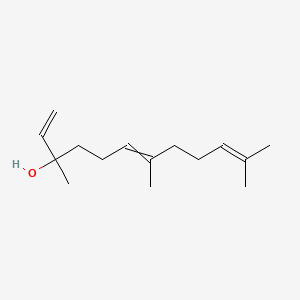
1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-
|
| Molecular Formula | C15H26O | |
| IUPAC Name* |
3,7,11-trimethyldodeca-1,6,10-trien-3-ol
|
|
| SMILES |
CC(=CCCC(=CCCC(C)(C=C)O)C)C
|
|
| InChI |
InChI=1S/C15H26O/c1-6-15(5,16)12-8-11-14(4)10-7-9-13(2)3/h6,9,11,16H,1,7-8,10,12H2,2-5H3
|
|
| InChIKey |
FQTLCLSUCSAZDY-UHFFFAOYSA-N
|
|
| Synonyms |
NEROLIDOL; Stirrup; trans nerolidol; Peruviol; (+)-Nerolidol; 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-; (Z)-Nerolidol; Nerolidol (natural); Melaleucol; dextro-nerolidol; Humbertiol?; (+/-)-Nerolidol; Spectrum_001222; SpecPlus_000303; Spectrum2_001507; Spectrum3_001539; Spectrum4_001720; DSSTox_CID_2247; DSSTox_RID_76528; DSSTox_GSID_22247; KBioGR_002080; KBioSS_001702; DivK1c_006399; SPBio_001553; CHEMBL3182436; DTXSID3022247; KBio1_001343; KBio2_001702; KBio2_004270; KBio2_006838; KBio3_002458; Tox21_301382; ( inverted exclamation markA)-Nerolidol; NCGC00255198-01; NCGC00344526-01; CAS-7212-44-4; DB-070076; FT-0600404; FT-0623906; FT-0773978; 3-Hydroxy-3,7,11-trimethyl-1,6,10-dodectriene
|
|
| CAS | 7212-44-4 | |
| PubChem CID | 8888 | |
| ChEMBL ID | CHEMBL3182436 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 222.37 | ALogp: | 4.6 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 16 | QED Weighted: | 0.606 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.496 | MDCK Permeability: | 0.00001850 |
| Pgp-inhibitor: | 0.741 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.877 |
| 30% Bioavailability (F30%): | 0.829 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.175 | Plasma Protein Binding (PPB): | 92.52% |
| Volume Distribution (VD): | 1.97 | Fu: | 6.93% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.377 | CYP1A2-substrate: | 0.149 |
| CYP2C19-inhibitor: | 0.622 | CYP2C19-substrate: | 0.829 |
| CYP2C9-inhibitor: | 0.244 | CYP2C9-substrate: | 0.887 |
| CYP2D6-inhibitor: | 0.217 | CYP2D6-substrate: | 0.074 |
| CYP3A4-inhibitor: | 0.746 | CYP3A4-substrate: | 0.239 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.808 | Half-life (T1/2): | 0.188 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.656 |
| Drug-inuced Liver Injury (DILI): | 0.019 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.008 | Maximum Recommended Daily Dose: | 0.022 |
| Skin Sensitization: | 0.928 | Carcinogencity: | 0.251 |
| Eye Corrosion: | 0.186 | Eye Irritation: | 0.973 |
| Respiratory Toxicity: | 0.015 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001606 | 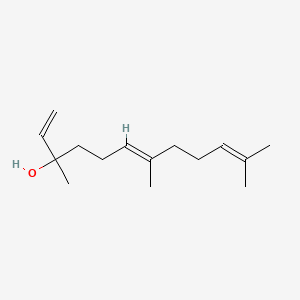 |
1.000 | D05XQE | 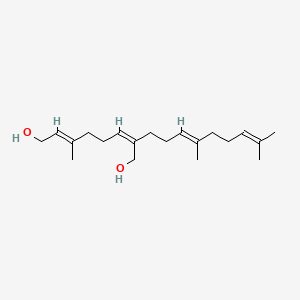 |
0.417 | ||
| ENC001716 | 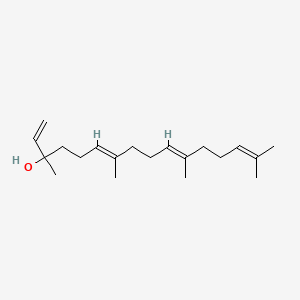 |
0.750 | D09XWD | 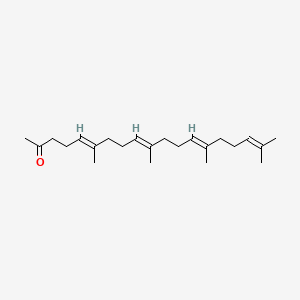 |
0.372 | ||
| ENC001467 | 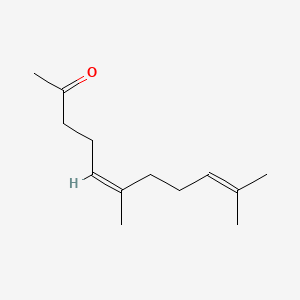 |
0.549 | D03VFL | 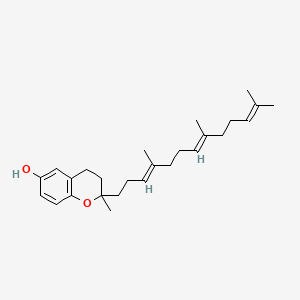 |
0.333 | ||
| ENC001462 | 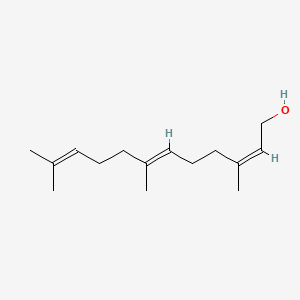 |
0.545 | D0M1PQ | 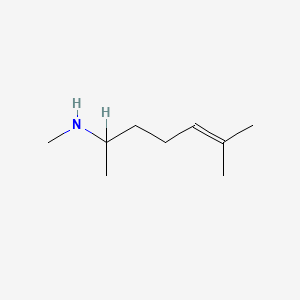 |
0.283 | ||
| ENC001096 | 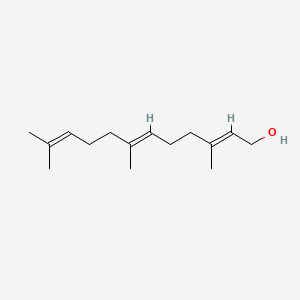 |
0.545 | D06BLQ | 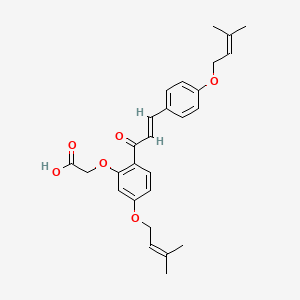 |
0.175 | ||
| ENC002570 | 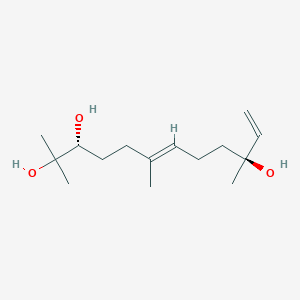 |
0.544 | D05PLH | 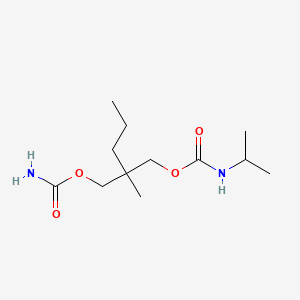 |
0.171 | ||
| ENC001564 | 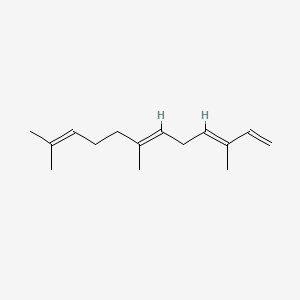 |
0.519 | D0X7XG | 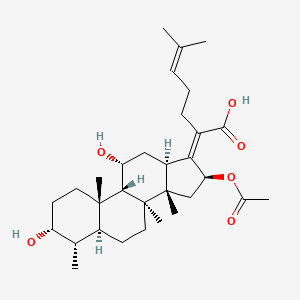 |
0.165 | ||
| ENC001664 | 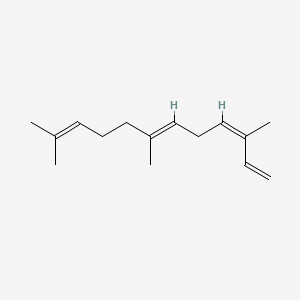 |
0.519 | D0S7WX |  |
0.165 | ||
| ENC001717 |  |
0.518 | D0Z5BC |  |
0.164 | ||
| ENC002413 |  |
0.518 | D09ANG |  |
0.160 | ||