NPs Basic Information
|
Name |
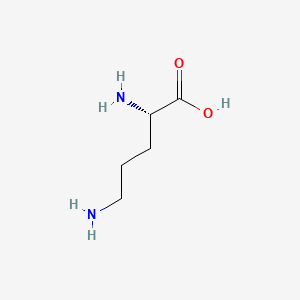
L-ornithine
|
| Molecular Formula | C5H12N2O2 | |
| IUPAC Name* |
(2S)-2,5-diaminopentanoic acid
|
|
| SMILES |
C(C[C@@H](C(=O)O)N)CN
|
|
| InChI |
InChI=1S/C5H12N2O2/c6-3-1-2-4(7)5(8)9/h4H,1-3,6-7H2,(H,8,9)/t4-/m0/s1
|
|
| InChIKey |
AHLPHDHHMVZTML-BYPYZUCNSA-N
|
|
| Synonyms |
L-ornithine; ornithine; 70-26-8; (S)-2,5-Diaminopentanoic acid; (S)-Ornithine; (S)-2,5-diaminovaleric acid; Ornithine [INN]; (2S)-2,5-diaminopentanoic acid; L-Norvaline, 5-amino-; (S)-alpha,delta-Diaminovaleric acid; Ornithinum [Latin]; Ornitina [Spanish]; Ornithine (VAN); (+)-S-Ornithine; (S)-2,5-Diaminopentanoate; Ornithine, (L)-Isomer; Pentanoic acid, 2,5-diamino-, (S)-; ORNITHINE, L-; L-Ornithine, HCl; BRN 1722298; L-(-)-Ornithine; CHEBI:15729; NSC-758894; E524N2IXA3; Ornithine (INN); L( )-Ornithine; 5-amino-L-Norvaline; Ornithinum; Ornitina; ORN; L(-)-Ornithine; EINECS 200-731-7; UNII-E524N2IXA3; levo-ornithine; 1hqg; 1lah; 3jdw; 25104-12-5; L-Ornithine (9CI); 5-diaminopentanoic acid; ORNITHINE [MI]; ORNITHINE [INCI]; (S)-a,d-Diaminovalerate; Ornithine, L- (8CI); bmse000162; ORNITHINE [MART.]; ORNITHINE [WHO-DD]; SCHEMBL8579; GTPL725; (S)-a,d-Diaminovaleric acid; 4-04-00-02644 (Beilstein Handbook Reference); Pentanoic acid, 2,5-diamino; CHEMBL446143; alpha, delta-diaminovaleric acid; DTXSID00883219; L-Ornithine2,5-Diaminovalericacid; Pharmakon1600-01504524; HY-B1352; ZINC1532530; (R,S)-2,5-Diamino-pentanoic acid; BDBM50487430; L-Ornithine;2,5-Diaminovaleric acid; MFCD00242584; NSC758894; s4857; AKOS006239312; CS-4817; DB00129; NSC 758894; SMP2_000009; NCGC00263569-01; AC-13803; AS-80993; (S)-2; S4653; EN300-96942; C00077; D08302; LYSINE ACETATE IMPURITY E [EP IMPURITY]; 070O268; A866639; Q410198; W-104562; 8AB10027-4D34-488A-9F55-E86692CA2853
|
|
| CAS | 70-26-8 | |
| PubChem CID | 6262 | |
| ChEMBL ID | CHEMBL446143 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 132.16 | ALogp: | -4.4 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 89.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.486 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.295 | MDCK Permeability: | 0.00817485 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.014 |
| Human Intestinal Absorption (HIA): | 0.132 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.323 | Plasma Protein Binding (PPB): | 7.53% |
| Volume Distribution (VD): | 0.694 | Fu: | 93.97% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.009 | CYP1A2-substrate: | 0.04 |
| CYP2C19-inhibitor: | 0.027 | CYP2C19-substrate: | 0.055 |
| CYP2C9-inhibitor: | 0.009 | CYP2C9-substrate: | 0.119 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.28 |
| CYP3A4-inhibitor: | 0.008 | CYP3A4-substrate: | 0.035 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.328 | Half-life (T1/2): | 0.515 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.066 |
| Drug-inuced Liver Injury (DILI): | 0.008 | AMES Toxicity: | 0.317 |
| Rat Oral Acute Toxicity: | 0.139 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.476 | Carcinogencity: | 0.08 |
| Eye Corrosion: | 0.018 | Eye Irritation: | 0.09 |
| Respiratory Toxicity: | 0.294 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000123 | 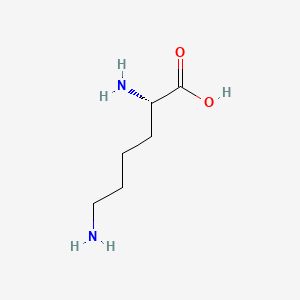 |
0.815 | D0F5DO | 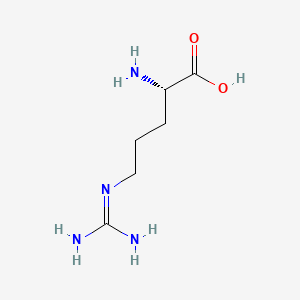 |
0.543 | ||
| ENC000550 | 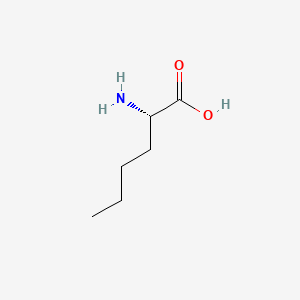 |
0.643 | D01JIA | 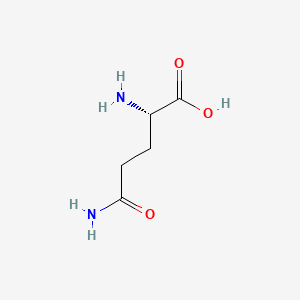 |
0.500 | ||
| ENC000795 |  |
0.545 | D01OPV | 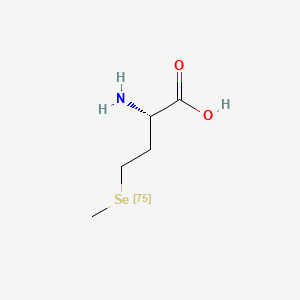 |
0.484 | ||
| ENC000142 | 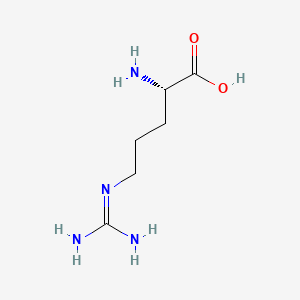 |
0.543 | D00ENY | 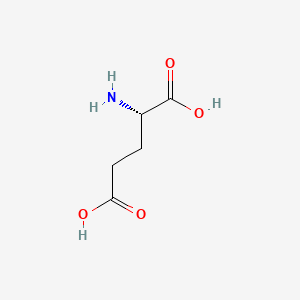 |
0.455 | ||
| ENC000539 | 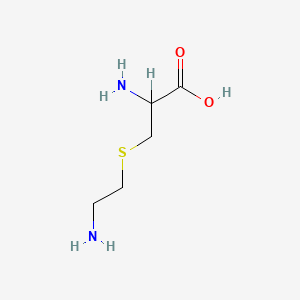 |
0.531 | D02UDJ | 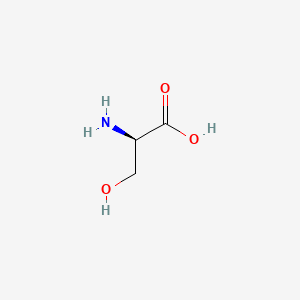 |
0.429 | ||
| ENC000760 | 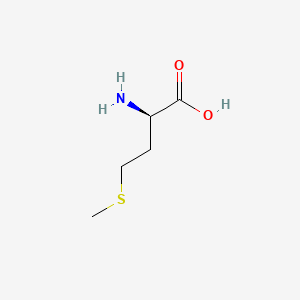 |
0.484 | D0P0QK | 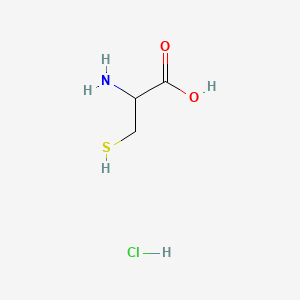 |
0.414 | ||
| ENC001215 | 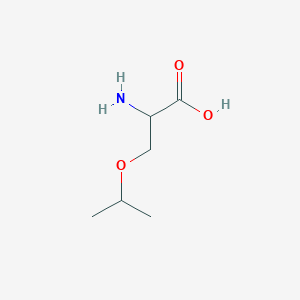 |
0.333 | D03CHT | 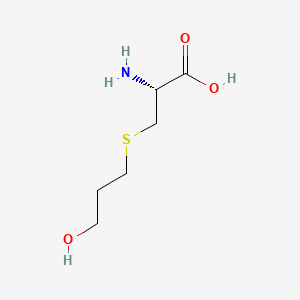 |
0.405 | ||
| ENC000937 | 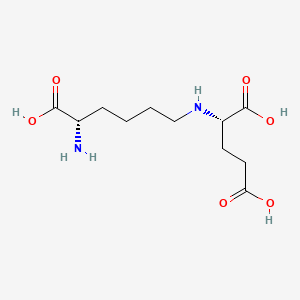 |
0.327 | D0X7JR | 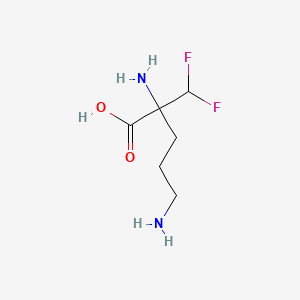 |
0.395 | ||
| ENC000306 | 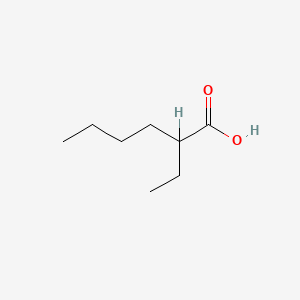 |
0.324 | D0FD0H | 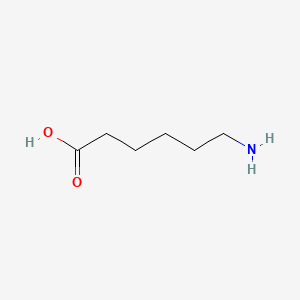 |
0.382 | ||
| ENC002789 | 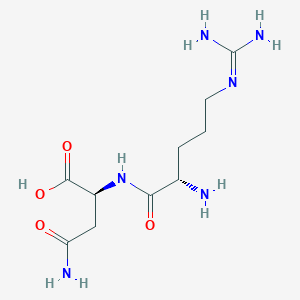 |
0.293 | D0X5SI | 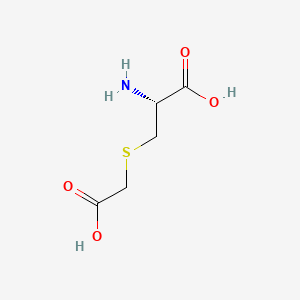 |
0.342 | ||