NPs Basic Information
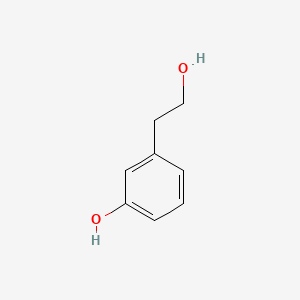
|
Name |
3-Hydroxyphenethyl alcohol
|
| Molecular Formula | C8H10O2 | |
| IUPAC Name* |
3-(2-hydroxyethyl)phenol
|
|
| SMILES |
C1=CC(=CC(=C1)O)CCO
|
|
| InChI |
InChI=1S/C8H10O2/c9-5-4-7-2-1-3-8(10)6-7/h1-3,6,9-10H,4-5H2
|
|
| InChIKey |
AMQIPHZFLIDOCB-UHFFFAOYSA-N
|
|
| Synonyms |
3-(2-Hydroxyethyl)phenol; 13398-94-2; 3-Hydroxyphenethyl alcohol; 2-(3-Hydroxyphenyl)ethanol; Benzeneethanol, 3-hydroxy-; Phenethyl alcohol, m-hydroxy-; 3-Hydroxyphenethylalcohol; m-Hydroxyphenethyl alcohol; m-Phenethyl alcohol; 3-(2-Hydroxy-ethyl)-phenol; 2D3F6MU88Z; MFCD00002895; NSC-101846; 3-Hydroxybenzeneethanol; UNII-2D3F6MU88Z; m-tyrosol; EINECS 236-485-2; 2-(3-hydroxyphenyl)-ethanol; 3-Hydroxyphenylmethyl carbinol; CHEMBL54498; SCHEMBL351675; DTXSID40158438; 2-(3-Hydroxyphenyl)ethanol, 99%; ACT08257; AMY41470; BCP30364; NSC101846; ZINC89223775; AKOS009156879; NSC 101846; DS-16080; SY011808; DB-004186; CS-0171132; FT-0691883; EN300-103529; J-006463; Q27254583; Benzeneethanol, 3-hydroxy-;2-(3-Hydroxyphenyl)ethanol;m-Hydroxyphenethyl alcohol;m-Phenethyl alcohol
|
|
| CAS | 13398-94-2 | |
| PubChem CID | 83404 | |
| ChEMBL ID | CHEMBL54498 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.16 | ALogp: | 1.2 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.647 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.255 | MDCK Permeability: | 0.00001890 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.12 | 20% Bioavailability (F20%): | 0.989 |
| 30% Bioavailability (F30%): | 0.994 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.137 | Plasma Protein Binding (PPB): | 26.54% |
| Volume Distribution (VD): | 2.881 | Fu: | 64.46% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.71 | CYP1A2-substrate: | 0.384 |
| CYP2C19-inhibitor: | 0.154 | CYP2C19-substrate: | 0.193 |
| CYP2C9-inhibitor: | 0.037 | CYP2C9-substrate: | 0.687 |
| CYP2D6-inhibitor: | 0.165 | CYP2D6-substrate: | 0.476 |
| CYP3A4-inhibitor: | 0.052 | CYP3A4-substrate: | 0.232 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.042 | Half-life (T1/2): | 0.899 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.048 | Human Hepatotoxicity (H-HT): | 0.064 |
| Drug-inuced Liver Injury (DILI): | 0.032 | AMES Toxicity: | 0.038 |
| Rat Oral Acute Toxicity: | 0.079 | Maximum Recommended Daily Dose: | 0.032 |
| Skin Sensitization: | 0.894 | Carcinogencity: | 0.176 |
| Eye Corrosion: | 0.922 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.035 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000003 | 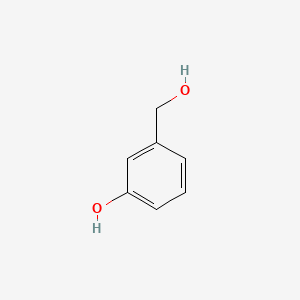 |
0.710 | D0O6IU | 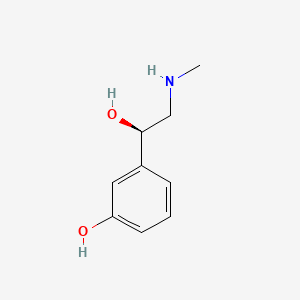 |
0.419 | ||
| ENC003374 | 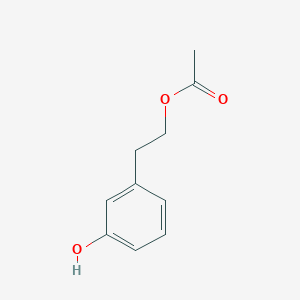 |
0.561 | D04EYC | 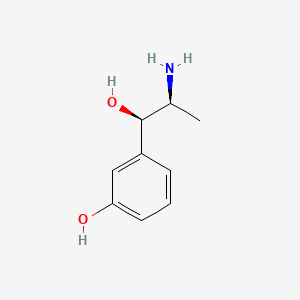 |
0.395 | ||
| ENC000350 | 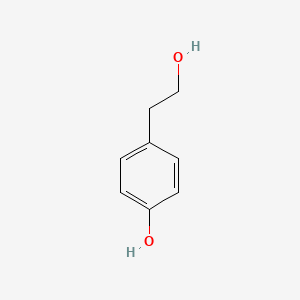 |
0.556 | D0S5LH | 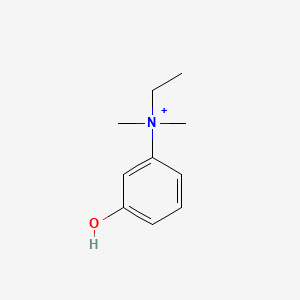 |
0.395 | ||
| ENC000128 | 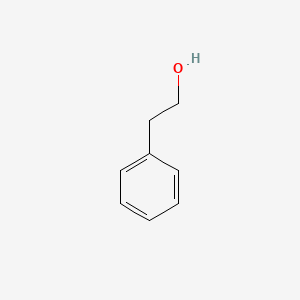 |
0.500 | D0T7OW | 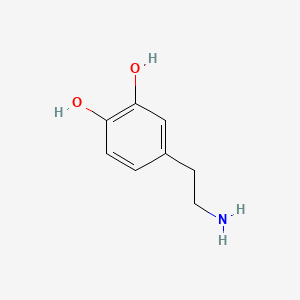 |
0.381 | ||
| ENC000507 | 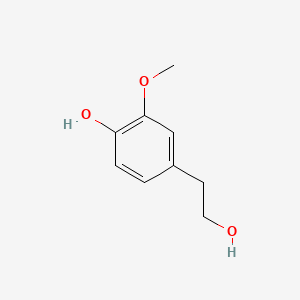 |
0.488 | D05OIS |  |
0.342 | ||
| ENC005498 | 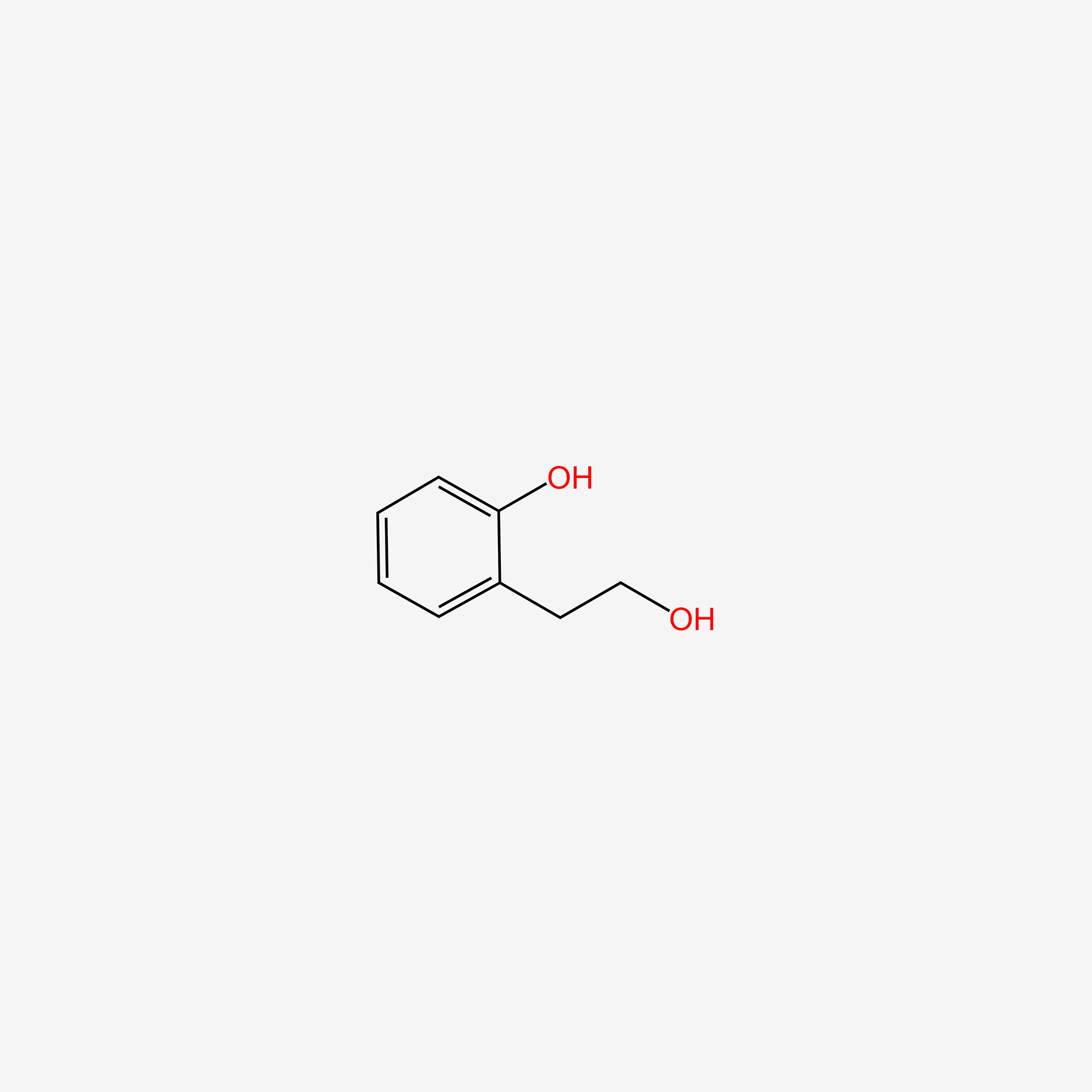 |
0.474 | D0K4MH | 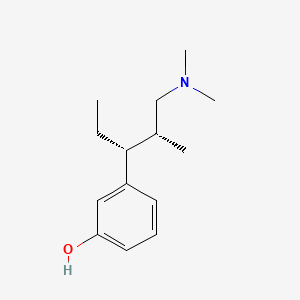 |
0.340 | ||
| ENC000754 | 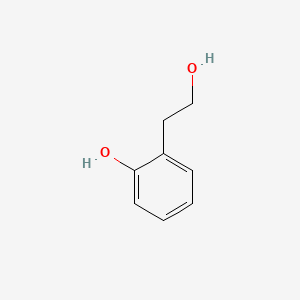 |
0.474 | D03UOT |  |
0.316 | ||
| ENC001049 | 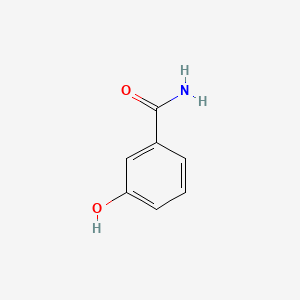 |
0.410 | D02JIS | 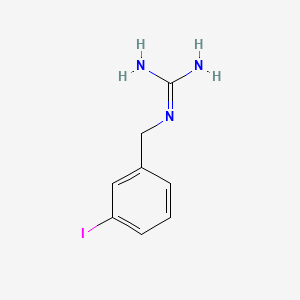 |
0.298 | ||
| ENC000394 | 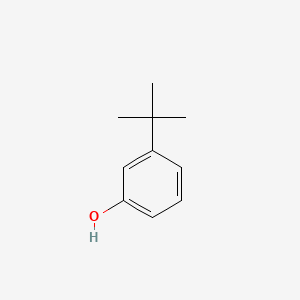 |
0.390 | D0P9AC |  |
0.295 | ||
| ENC000985 |  |
0.375 | D0S5YC |  |
0.288 | ||