NPs Basic Information
|
Name |
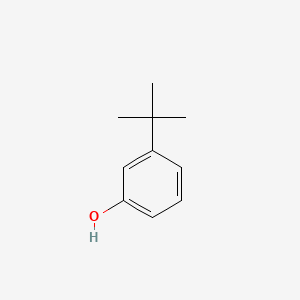
3-tert-Butylphenol
|
| Molecular Formula | C10H14O | |
| IUPAC Name* |
3-tert-butylphenol
|
|
| SMILES |
CC(C)(C)C1=CC(=CC=C1)O
|
|
| InChI |
InChI=1S/C10H14O/c1-10(2,3)8-5-4-6-9(11)7-8/h4-7,11H,1-3H3
|
|
| InChIKey |
CYEKUDPFXBLGHH-UHFFFAOYSA-N
|
|
| Synonyms |
3-tert-Butylphenol; 585-34-2; m-tert-Butylphenol; 3-(tert-butyl)phenol; Phenol, 3-(1,1-dimethylethyl)-; 3-t-Butylphenol; 3-tert-butyl-phenol; Phenol, m-tert-butyl-; CHEBI:34348; 2382U55WN2; MFCD00002300; UNII-2382U55WN2; 3-tertbutylphenol; 3-tert.butylphenol; EINECS 209-553-4; m-tert.-butylphenol; 3-tert.-butylphenol; meta-tert-butylphenol; 3-tert-Butyl phenol; 3-tert-Butylphenol;; Phenol, 3-tert-butyl-; ST50824235; 3-tert-butyl-hydroxybenzene; 3-tert-Butylphenol, 99%; DSSTox_CID_24825; DSSTox_RID_80506; DSSTox_GSID_44825; SCHEMBL50933; BIDD:ER0561; CHEMBL224899; 3-(1,1-dimethylethyl)-phenol; DTXSID9044825; CYEKUDPFXBLGHH-UHFFFAOYSA-; ZINC2012740; Tox21_301686; 3-(1,1-DIMETHYLETHYL)PHENOL; 5-TERT-BUTYL-1-HYDROXYBENZENE; AKOS000120521; NCGC00256058-01; AC-26474; AS-14546; CAS-585-34-2; B0731; CS-0015724; FT-0616395; EN300-20961; O11053; A831909; AE-562/43460593; Q-200396; Q27116004
|
|
| CAS | 585-34-2 | |
| PubChem CID | 11450 | |
| ChEMBL ID | CHEMBL224899 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 150.22 | ALogp: | 3.3 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.6 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.557 | MDCK Permeability: | 0.00002120 |
| Pgp-inhibitor: | 0.025 | Pgp-substrate: | 0.018 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.503 |
| 30% Bioavailability (F30%): | 0.64 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.47 | Plasma Protein Binding (PPB): | 90.78% |
| Volume Distribution (VD): | 3.412 | Fu: | 13.56% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.965 | CYP1A2-substrate: | 0.841 |
| CYP2C19-inhibitor: | 0.823 | CYP2C19-substrate: | 0.619 |
| CYP2C9-inhibitor: | 0.529 | CYP2C9-substrate: | 0.895 |
| CYP2D6-inhibitor: | 0.807 | CYP2D6-substrate: | 0.723 |
| CYP3A4-inhibitor: | 0.176 | CYP3A4-substrate: | 0.37 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.363 | Half-life (T1/2): | 0.763 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.052 |
| Drug-inuced Liver Injury (DILI): | 0.029 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.33 | Maximum Recommended Daily Dose: | 0.248 |
| Skin Sensitization: | 0.817 | Carcinogencity: | 0.063 |
| Eye Corrosion: | 0.981 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.363 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000898 |  |
0.548 | D0S5LH |  |
0.452 | ||
| ENC000500 |  |
0.500 | D06YPU | 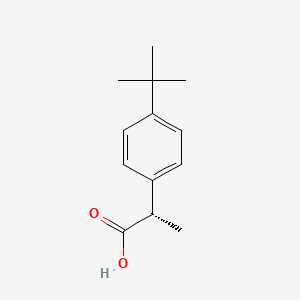 |
0.388 | ||
| ENC000185 | 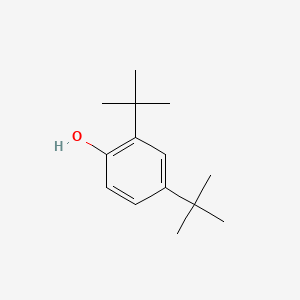 |
0.457 | D04EYC | 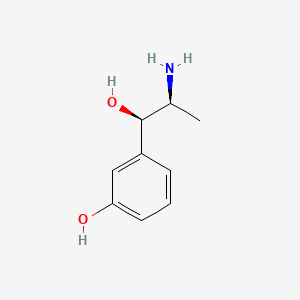 |
0.386 | ||
| ENC000744 | 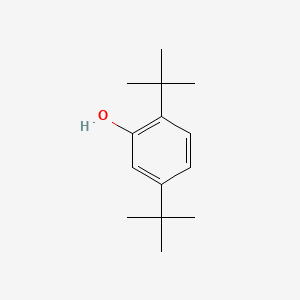 |
0.457 | D0O6IU | 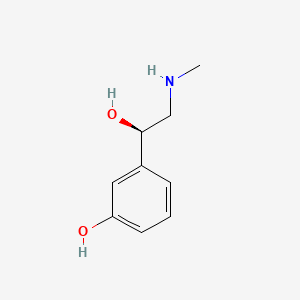 |
0.378 | ||
| ENC005113 | 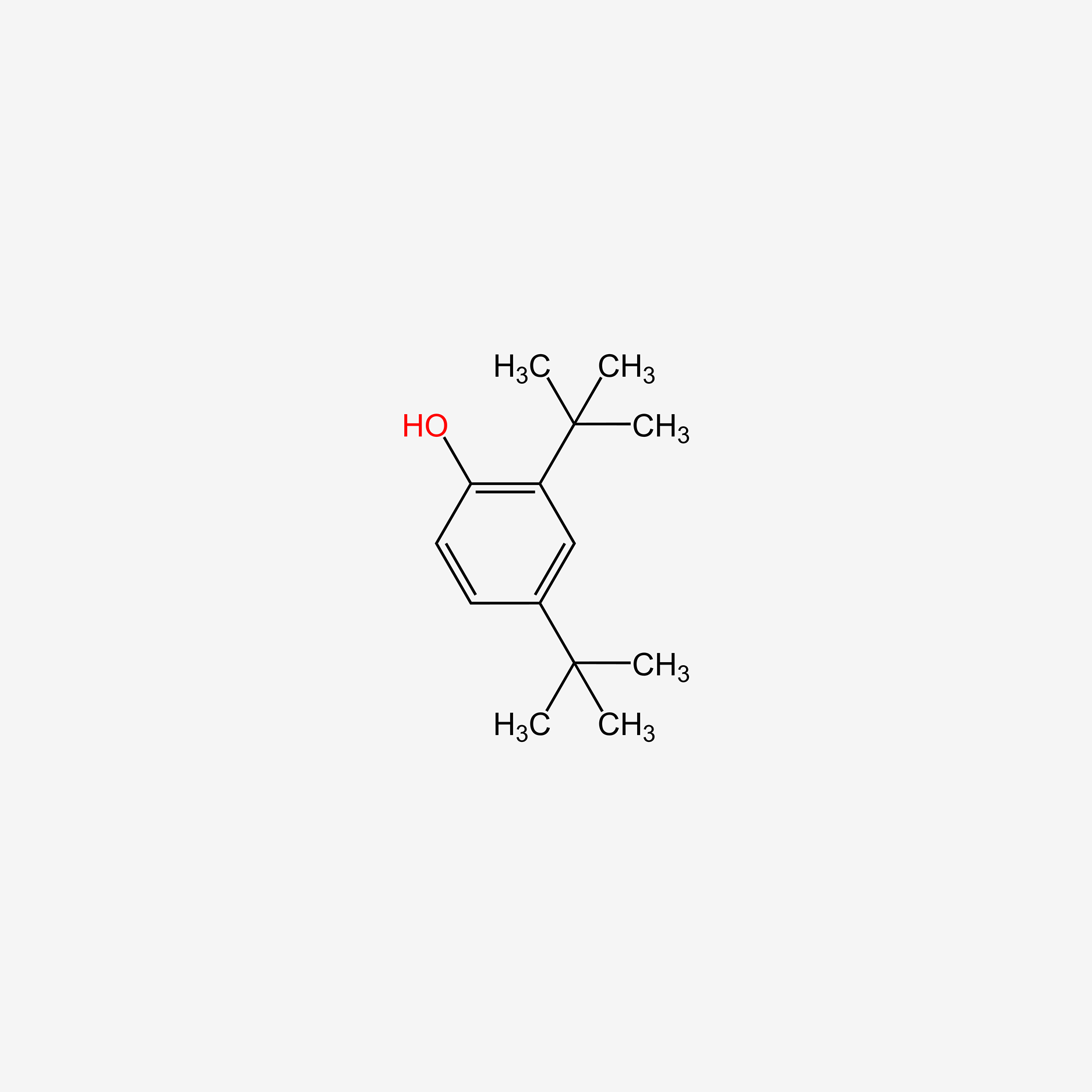 |
0.457 | D0K4MH | 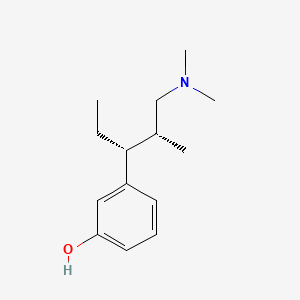 |
0.358 | ||
| ENC000695 | 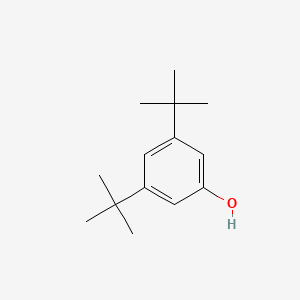 |
0.426 | D0X4ZR | 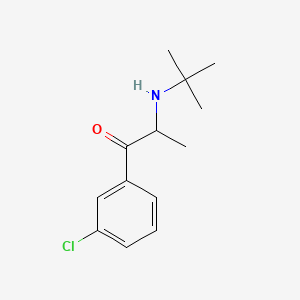 |
0.340 | ||
| ENC000003 | 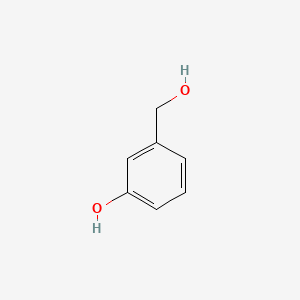 |
0.421 | D0S5YC | 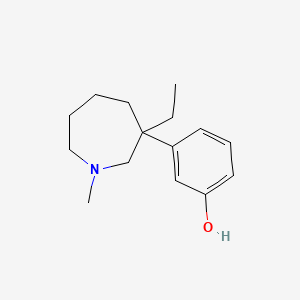 |
0.305 | ||
| ENC000152 | 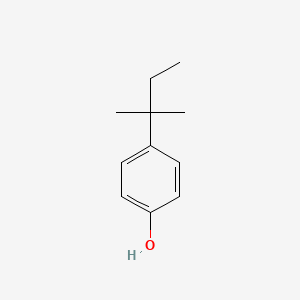 |
0.419 | D0K5CB | 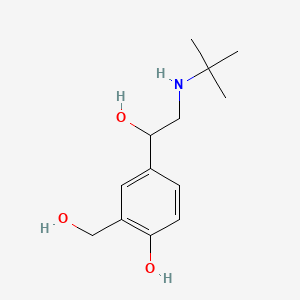 |
0.298 | ||
| ENC001049 | 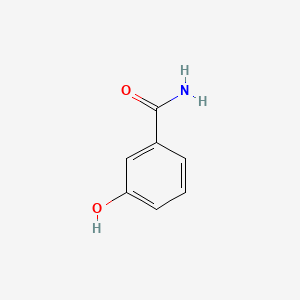 |
0.400 | D02ZJI | 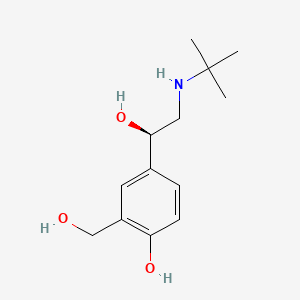 |
0.298 | ||
| ENC000611 | 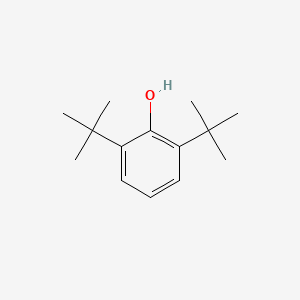 |
0.396 | D0G1OZ | 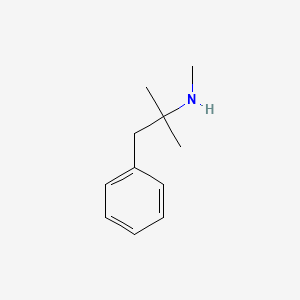 |
0.292 | ||