NPs Basic Information
|
Name |
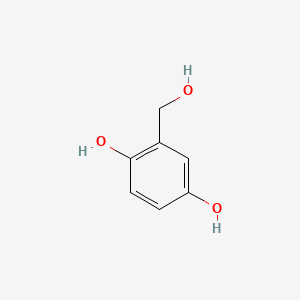
Gentisyl alcohol
|
| Molecular Formula | C7H8O3 | |
| IUPAC Name* |
2-(hydroxymethyl)benzene-1,4-diol
|
|
| SMILES |
C1=CC(=C(C=C1O)CO)O
|
|
| InChI |
InChI=1S/C7H8O3/c8-4-5-3-6(9)1-2-7(5)10/h1-3,8-10H,4H2
|
|
| InChIKey |
PUZSUVGRVHEUQO-UHFFFAOYSA-N
|
|
| Synonyms |
Gentisyl alcohol; 2-(hydroxymethyl)benzene-1,4-diol; 495-08-9; 2,5-Dihydroxybenzyl alcohol; Salirepol; 3,6-Dihydroxybenzyl alcohol; 2-(hydroxymethyl)-1,4-benzenediol; gentisin alcohol; 1,4-Benzenediol, 2-(hydroxymethyl)-; T8T2WY38GH; CHEBI:5325; UNII-T8T2WY38GH; GENTISYL ALCOHOL [MI]; SCHEMBL829492; CHEMBL448800; PUZSUVGRVHEUQO-UHFFFAOYSA-; DTXSID60197804; ZINC900388; BE-53594B; MFCD06202616; 2-(hydroxymethyl)-benzene-1,4-diol; AKOS006293496; BS-1180; CS-0378114; E88042; EN300-1843206; A819991; Q27106721; 2,5-DIHYDROXYBENZYL ALCOHOL, HYDROXYMETHYL-HYDROCHINONE; 2-(hydroxymethyl)benzene-1,4-diol;3,5-Dihydroxybenzyl Alcohol; 2,5-Dihydroxybenzyl alcohol; Gentisyl alcohol; Salirepol; Gentisin alcohol
|
|
| CAS | 495-08-9 | |
| PubChem CID | 188287 | |
| ChEMBL ID | CHEMBL448800 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 140.14 | ALogp: | 0.7 |
| HBD: | 3 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 60.7 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.51 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.559 | MDCK Permeability: | 0.00000914 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.032 | 20% Bioavailability (F20%): | 0.939 |
| 30% Bioavailability (F30%): | 0.922 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.032 | Plasma Protein Binding (PPB): | 29.29% |
| Volume Distribution (VD): | 0.966 | Fu: | 67.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.24 | CYP1A2-substrate: | 0.23 |
| CYP2C19-inhibitor: | 0.05 | CYP2C19-substrate: | 0.065 |
| CYP2C9-inhibitor: | 0.017 | CYP2C9-substrate: | 0.715 |
| CYP2D6-inhibitor: | 0.075 | CYP2D6-substrate: | 0.791 |
| CYP3A4-inhibitor: | 0.035 | CYP3A4-substrate: | 0.15 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 14.215 | Half-life (T1/2): | 0.944 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.022 | Human Hepatotoxicity (H-HT): | 0.031 |
| Drug-inuced Liver Injury (DILI): | 0.045 | AMES Toxicity: | 0.625 |
| Rat Oral Acute Toxicity: | 0.527 | Maximum Recommended Daily Dose: | 0.018 |
| Skin Sensitization: | 0.917 | Carcinogencity: | 0.359 |
| Eye Corrosion: | 0.255 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.113 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002875 | 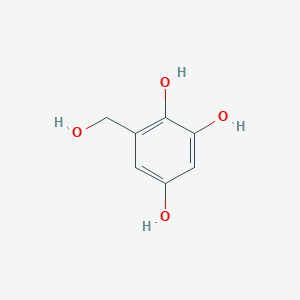 |
0.514 | D02ZJI | 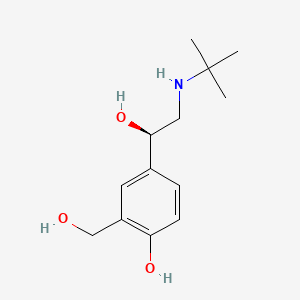 |
0.440 | ||
| ENC000097 | 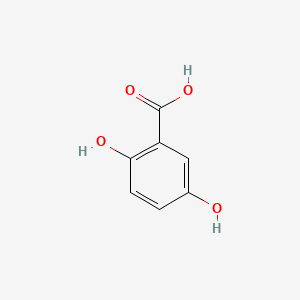 |
0.514 | D0K5CB | 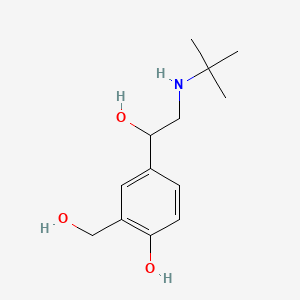 |
0.440 | ||
| ENC000696 | 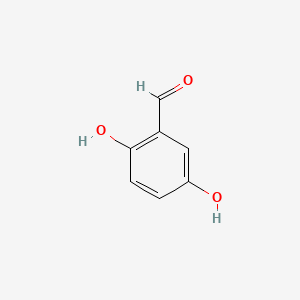 |
0.500 | D07MOX | 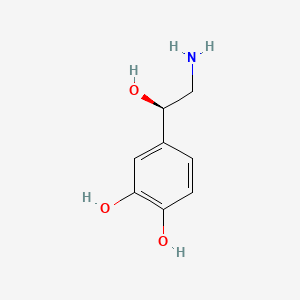 |
0.405 | ||
| ENC000069 | 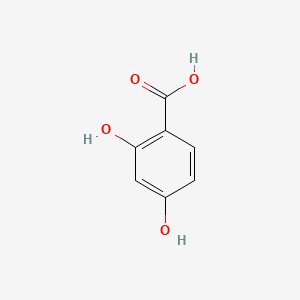 |
0.474 | D0YF3X | 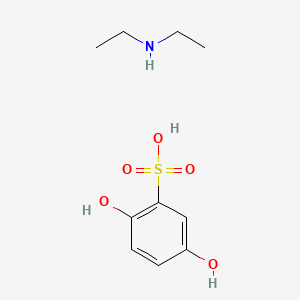 |
0.392 | ||
| ENC000344 | 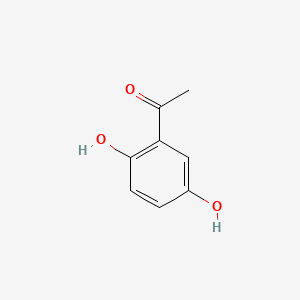 |
0.474 | D0T7OW | 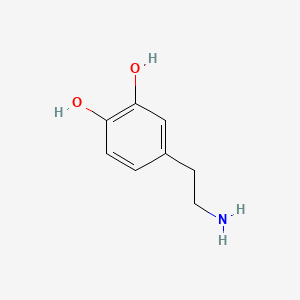 |
0.390 | ||
| ENC002464 | 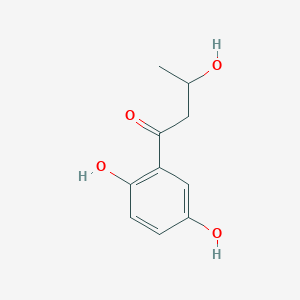 |
0.455 | D04PHC | 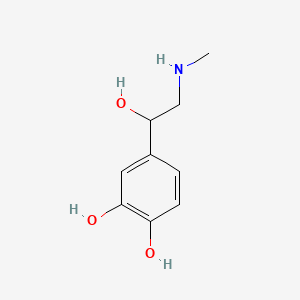 |
0.378 | ||
| ENC000500 | 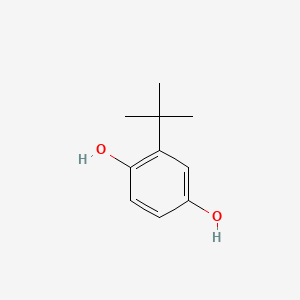 |
0.450 | D08HVR | 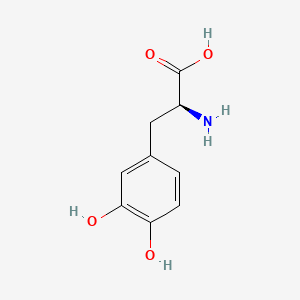 |
0.362 | ||
| ENC000003 | 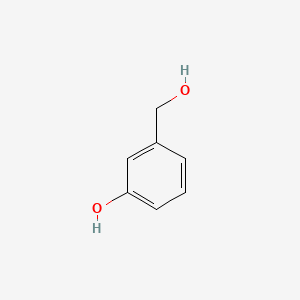 |
0.444 | D03UOT |  |
0.361 | ||
| ENC000734 | 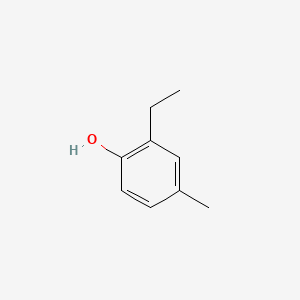 |
0.421 | D0V9EN |  |
0.348 | ||
| ENC002506 | 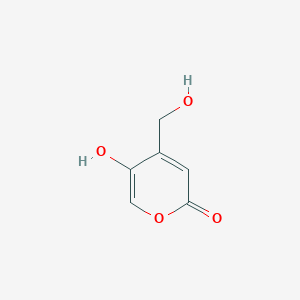 |
0.421 | D0BA6T | 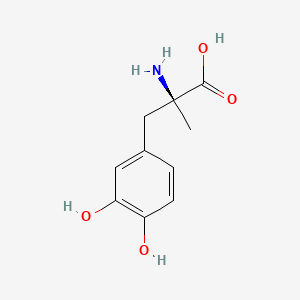 |
0.347 | ||