NPs Basic Information
|
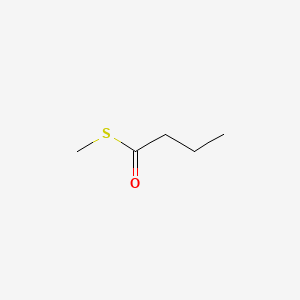
Name |
S-Methyl butanethioate
|
| Molecular Formula | C5H10OS | |
| IUPAC Name* |
S-methyl butanethioate
|
|
| SMILES |
CCCC(=O)SC
|
|
| InChI |
InChI=1S/C5H10OS/c1-3-4-5(6)7-2/h3-4H2,1-2H3
|
|
| InChIKey |
GRLJIIJNZJVMGP-UHFFFAOYSA-N
|
|
| Synonyms |
Methyl thiobutyrate; 2432-51-1; S-Methyl thiobutyrate; S-Methyl butanethioate; BUTANETHIOIC ACID, S-METHYL ESTER; s-Methyl thiobutanoate; Methanethiol butyrate; Methyl thiolbutyrate; Butyric acid, thio-, S-methyl ester; Methanethiol n-butyrate; FEMA No. 3310; Methyl thiobutanoate; Methane n-thiolbutyrate; 2P1E432MYZ; Thiobutyric Acid S-Methyl Ester; Methyl thiobutyrate (natural); EINECS 219-407-1; UNII-2P1E432MYZ; 1-(methylsulfanyl)butan-1-one; S-Methyl butanethioate #; S-(methyl thio) butyrate; S-Methyl thiobutanoate, 98%; SCHEMBL722981; DTXSID5047669; CHEBI:89255; FEMA 3310; METHYL THIOBUTYRATE [FCC]; METHYL THIOBUTYRATE [FHFI]; ZINC409240; Methyl thiobutyrate, >=97%, FG; MFCD00009872; AKOS015950859; Methyl thiobutyrate, natural, 98%, FG; LS-13066; DB-046399; CS-0323899; FT-0628812; M1818; D91533; 432M511; J-800454; Q-100312; Q27161441
|
|
| CAS | 2432-51-1 | |
| PubChem CID | 62444 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 118.2 | ALogp: | 1.5 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 42.4 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.553 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.324 | MDCK Permeability: | 0.00002440 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.023 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.996 | Plasma Protein Binding (PPB): | 58.32% |
| Volume Distribution (VD): | 0.771 | Fu: | 51.47% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.876 | CYP1A2-substrate: | 0.912 |
| CYP2C19-inhibitor: | 0.235 | CYP2C19-substrate: | 0.862 |
| CYP2C9-inhibitor: | 0.027 | CYP2C9-substrate: | 0.719 |
| CYP2D6-inhibitor: | 0.015 | CYP2D6-substrate: | 0.506 |
| CYP3A4-inhibitor: | 0.013 | CYP3A4-substrate: | 0.301 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.863 | Half-life (T1/2): | 0.899 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.026 | Human Hepatotoxicity (H-HT): | 0.049 |
| Drug-inuced Liver Injury (DILI): | 0.088 | AMES Toxicity: | 0.17 |
| Rat Oral Acute Toxicity: | 0.101 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.799 | Carcinogencity: | 0.749 |
| Eye Corrosion: | 0.94 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.155 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000018 |  |
0.435 | D0Y3KG |  |
0.257 | ||
| ENC000226 |  |
0.393 | D0ZK8H | 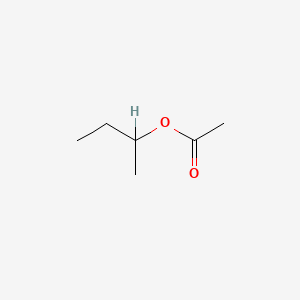 |
0.226 | ||
| ENC000713 | 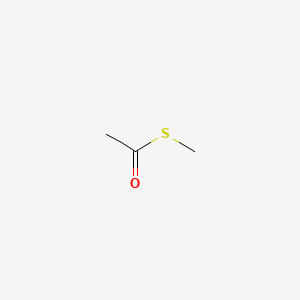 |
0.381 | D0EP8X | 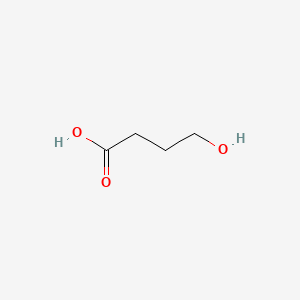 |
0.200 | ||
| ENC000685 | 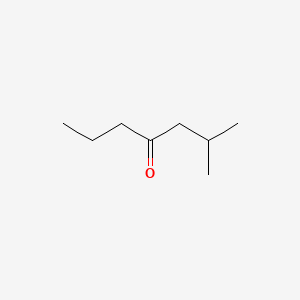 |
0.367 | D0U7BW | 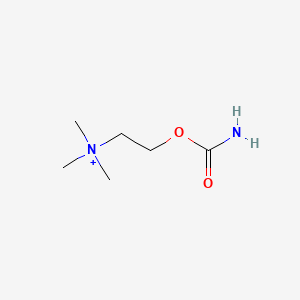 |
0.194 | ||
| ENC000250 | 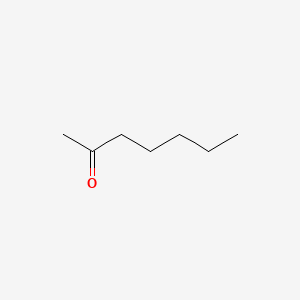 |
0.345 | D0Q9HF | 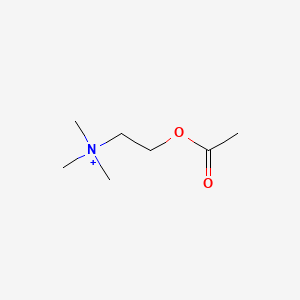 |
0.194 | ||
| ENC000232 | 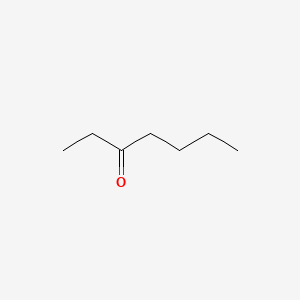 |
0.345 | D0XB8P | 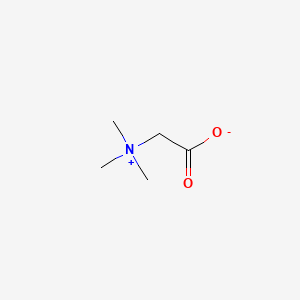 |
0.194 | ||
| ENC001004 | 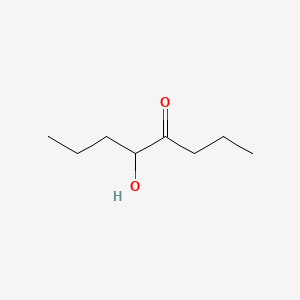 |
0.333 | D0OL6O | 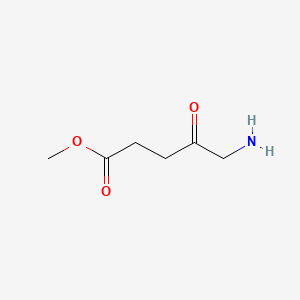 |
0.189 | ||
| ENC000738 | 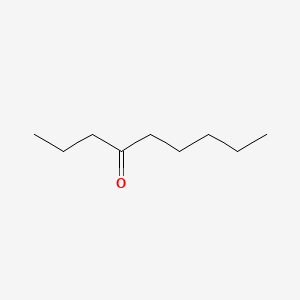 |
0.324 | D0Y4AW | 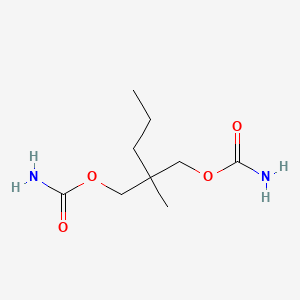 |
0.188 | ||
| ENC000245 | 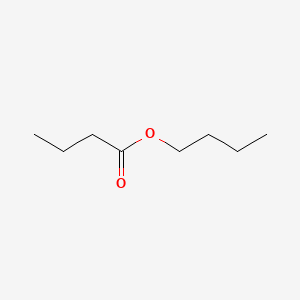 |
0.324 | D06VNK | 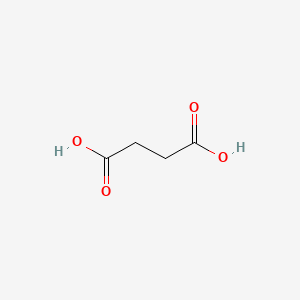 |
0.188 | ||
| ENC002437 | 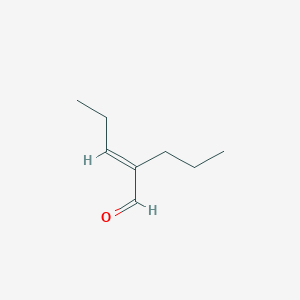 |
0.313 | D02KJX | 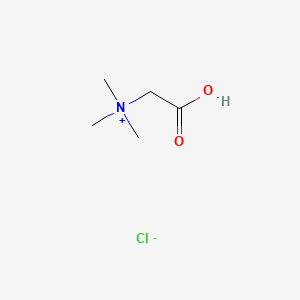 |
0.188 | ||