NPs Basic Information
|
Name |
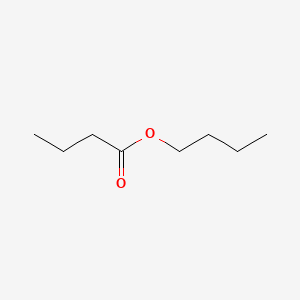
Butyl butyrate
|
| Molecular Formula | C8H16O2 | |
| IUPAC Name* |
butyl butanoate
|
|
| SMILES |
CCCCOC(=O)CCC
|
|
| InChI |
InChI=1S/C8H16O2/c1-3-5-7-10-8(9)6-4-2/h3-7H2,1-2H3
|
|
| InChIKey |
XUPYJHCZDLZNFP-UHFFFAOYSA-N
|
|
| Synonyms |
Butyl butyrate; 109-21-7; Butyl butanoate; n-Butyl butyrate; Butanoic acid, butyl ester; n-Butyl butanoate; Butyric Acid Butyl Ester; 1-Butyl butyrate; Butyl butylate; N-BUTYL N-BUTYRATE; Butyric acid, butyl ester; Butyl n-butyrate; Butylbutyrate; n-Butyl n-butanoate; FEMA No. 2186; butryl butyrate; NSC 8458; Butyric acid n-butyl ester; Butyl ester of butanoic acid; n-Butyric acid n-butyl ester; 1BHV00T1M4; NSC-8458; WE(4:0/4:0); Butyl butyrate (natural); CCRIS 6551; butanoic acid butyl ester; n-BUTYL-n-BUTYRATE; EINECS 203-656-8; BRN 1747101; UNII-1BHV00T1M4; AI3-06125; Butyric acid butyl; Butyl Butyrate Natural; Butyl butyrate, 98%; N-BUTYL-BUTYRATE; n-butyric n-butyl ester; Butyric acid-butyl ester; BUTYL BUTYRATE [FCC]; 4-02-00-00789 (Beilstein Handbook Reference); WLN: 4OV3; BUTYL BUTYRATE [FHFI]; SCHEMBL113994; DTXSID7041702; CHEBI:87429; FEMA 2186; N-BUTYL N-BUTYRATE [MI]; NSC8458; Butyl butyrate, analytical standard; ZINC1586750; LMFA07010418; MFCD00009450; Butyl butyrate, >=98%, FCC, FG; AKOS009165857; LS-13508; DB-003606; B0757; Butyl butyrate, natural, >=98%, FCC, FG; FT-0654703; EN300-52980; A801979; J-002246; J-519960; Q2212471
|
|
| CAS | 109-21-7 | |
| PubChem CID | 7983 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 144.21 | ALogp: | 2.2 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.438 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.303 | MDCK Permeability: | 0.00003230 |
| Pgp-inhibitor: | 0.06 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.349 |
| 30% Bioavailability (F30%): | 0.816 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.969 | Plasma Protein Binding (PPB): | 75.59% |
| Volume Distribution (VD): | 0.568 | Fu: | 36.60% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.978 | CYP1A2-substrate: | 0.677 |
| CYP2C19-inhibitor: | 0.704 | CYP2C19-substrate: | 0.676 |
| CYP2C9-inhibitor: | 0.322 | CYP2C9-substrate: | 0.726 |
| CYP2D6-inhibitor: | 0.03 | CYP2D6-substrate: | 0.203 |
| CYP3A4-inhibitor: | 0.049 | CYP3A4-substrate: | 0.2 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.991 | Half-life (T1/2): | 0.836 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.072 | Human Hepatotoxicity (H-HT): | 0.022 |
| Drug-inuced Liver Injury (DILI): | 0.114 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.075 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.77 | Carcinogencity: | 0.24 |
| Eye Corrosion: | 0.979 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.136 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000655 | 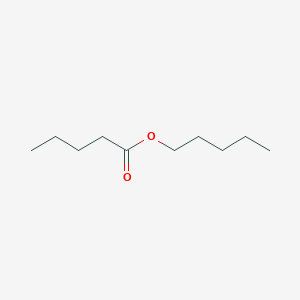 |
0.667 | D0AY9Q | 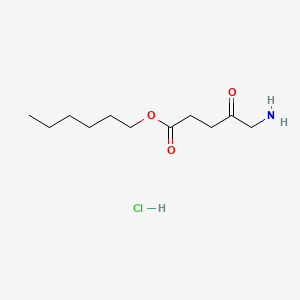 |
0.438 | ||
| ENC000226 | 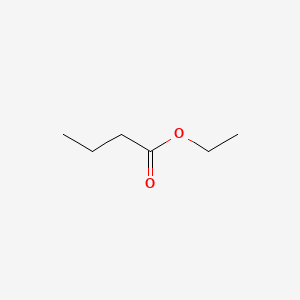 |
0.600 | D0Y3KG | 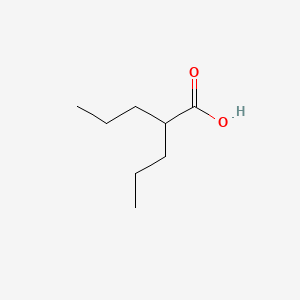 |
0.325 | ||
| ENC000371 | 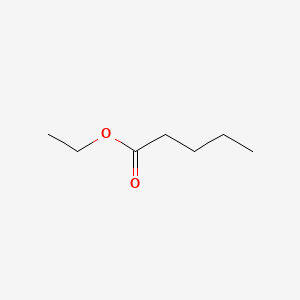 |
0.594 | D01QLH | 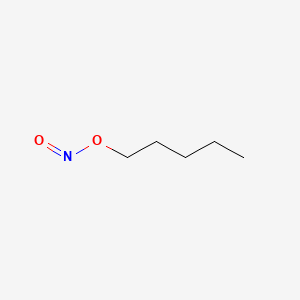 |
0.324 | ||
| ENC000231 | 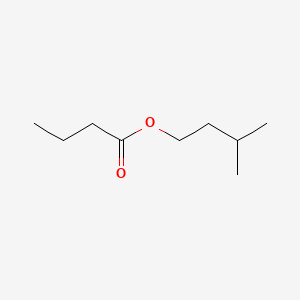 |
0.556 | D06ORU | 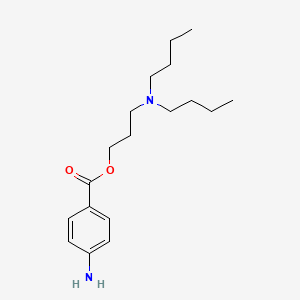 |
0.290 | ||
| ENC000718 | 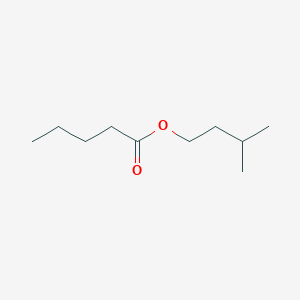 |
0.553 | D0H2SY | 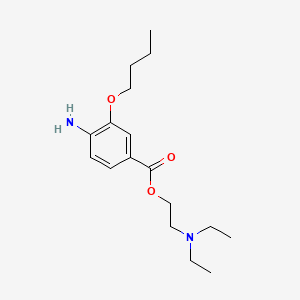 |
0.275 | ||
| ENC000602 | 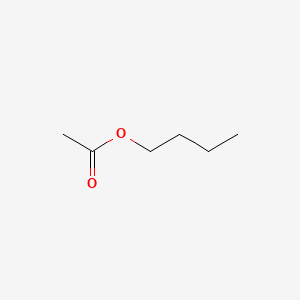 |
0.548 | D0Q7ZQ | 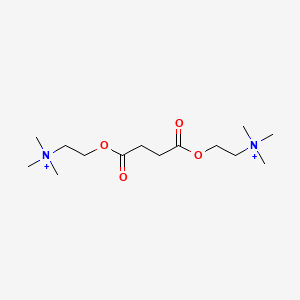 |
0.274 | ||
| ENC000742 | 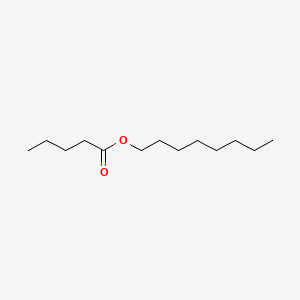 |
0.533 | D08HQK | 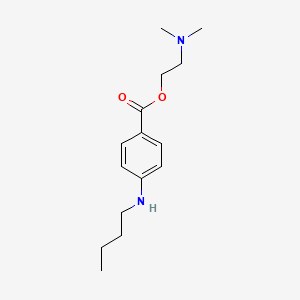 |
0.270 | ||
| ENC000773 | 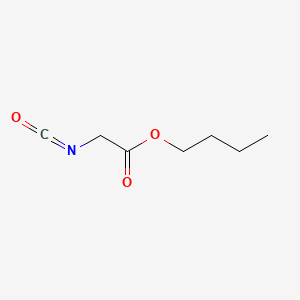 |
0.500 | D02HXS | 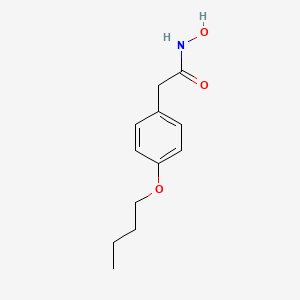 |
0.263 | ||
| ENC000738 | 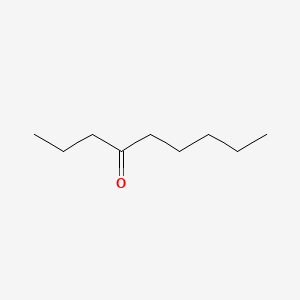 |
0.500 | D0NU2H | 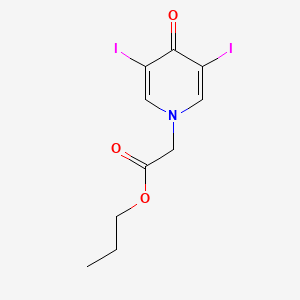 |
0.250 | ||
| ENC000645 | 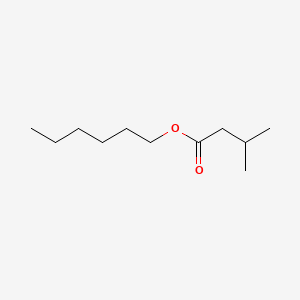 |
0.476 | D0Y4AW | 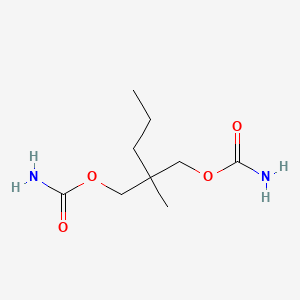 |
0.245 | ||