NPs Basic Information
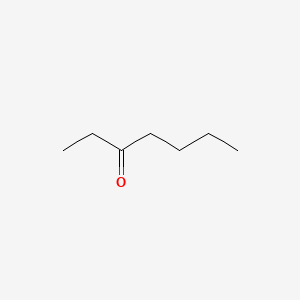
|
Name |
3-Heptanone
|
| Molecular Formula | C7H14O | |
| IUPAC Name* |
heptan-3-one
|
|
| SMILES |
CCCCC(=O)CC
|
|
| InChI |
InChI=1S/C7H14O/c1-3-5-6-7(8)4-2/h3-6H2,1-2H3
|
|
| InChIKey |
NGAZZOYFWWSOGK-UHFFFAOYSA-N
|
|
| Synonyms |
3-Heptanone; Heptan-3-one; 106-35-4; Butyl ethyl ketone; n-Butyl ethyl ketone; Aethylbutylketon; Ethylbutylcetone; Ethyl n-butyl ketone; ETHYL BUTYL KETONE; Ethylbutylketon; Etilbutilchetone; Ethyl-n-butyl ketone; Eptan-3-one; Heptan-3-on; Hexanone, methyl-; FEMA No. 2545; Ethyl butyl ketone 3-Heptanone; Aethylbutylketon [German]; NSC 8448; n-Heptan-3-one; Heptan-3-on [Dutch, German]; n-C4H9COC2H5; 10GA6SR3AT; NSC-8448; Aethylbutylketon (german); Heptan-3-on (DUTCH, GERMAN); Ethylbutylketon [Dutch]; Eptan-3-one [Italian]; Ethylbutylcetone [French]; Etilbutilchetone [Italian]; HSDB 1816; EINECS 203-388-1; UNII-10GA6SR3AT; BRN 0506161; AI3-19684; 3-Oxoheptane; MFCD00009483; 3-Heptanone, 96%; 3-HEPTANONE [FCC]; 3-HEPTANONE [FHFI]; 3-HEPTANONE [HSDB]; 4-01-00-03321 (Beilstein Handbook Reference); SCHEMBL105902; WLN: 4V2; DTXSID2047438; Fehling's reagent II for sugars; 3-Heptanone, analytical standard; CHEBI:50139; FEMA 2545; NSC8448; ZINC1586745; BBL011476; LMFA12000047; STL146588; AKOS005721019; VS-02958; FT-0615773; H0038; EN300-72299; D90783; A801423; J-512587; Q1287838; Z406378226
|
|
| CAS | 106-35-4 | |
| PubChem CID | 7802 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 114.19 | ALogp: | 1.8 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.549 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.294 | MDCK Permeability: | 0.00002490 |
| Pgp-inhibitor: | 0.039 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.245 |
| 30% Bioavailability (F30%): | 0.167 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.997 | Plasma Protein Binding (PPB): | 51.68% |
| Volume Distribution (VD): | 0.691 | Fu: | 51.34% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.528 | CYP1A2-substrate: | 0.889 |
| CYP2C19-inhibitor: | 0.079 | CYP2C19-substrate: | 0.758 |
| CYP2C9-inhibitor: | 0.036 | CYP2C9-substrate: | 0.663 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.719 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.197 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.333 | Half-life (T1/2): | 0.879 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.026 | Human Hepatotoxicity (H-HT): | 0.082 |
| Drug-inuced Liver Injury (DILI): | 0.215 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.048 | Maximum Recommended Daily Dose: | 0.028 |
| Skin Sensitization: | 0.185 | Carcinogencity: | 0.043 |
| Eye Corrosion: | 0.879 | Eye Irritation: | 0.976 |
| Respiratory Toxicity: | 0.222 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001025 | 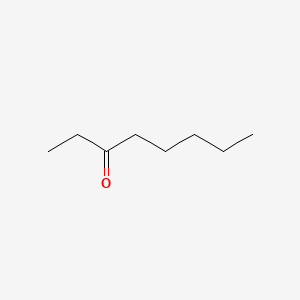 |
0.800 | D0Y3KG | 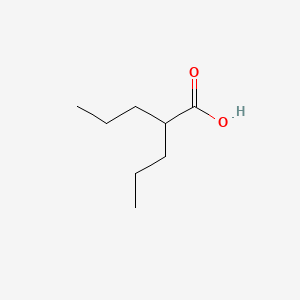 |
0.343 | ||
| ENC000371 | 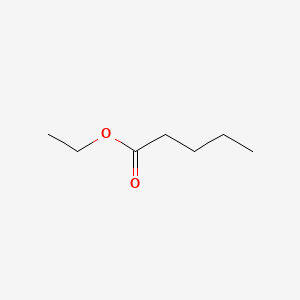 |
0.552 | D01QLH | 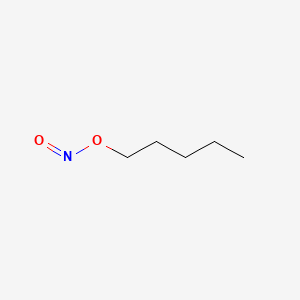 |
0.303 | ||
| ENC000738 | 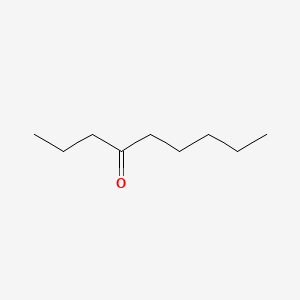 |
0.548 | D0AY9Q | 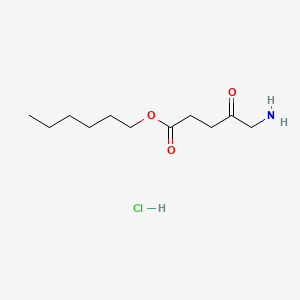 |
0.286 | ||
| ENC000487 | 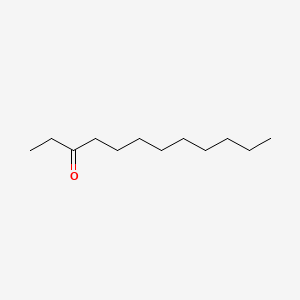 |
0.541 | D0EP8X | 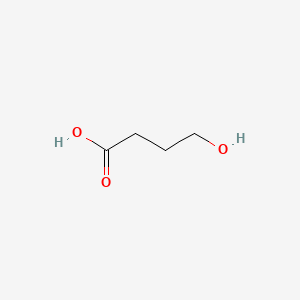 |
0.258 | ||
| ENC001253 | 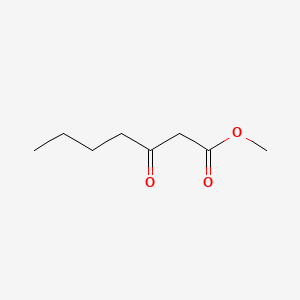 |
0.515 | D0FD0H | 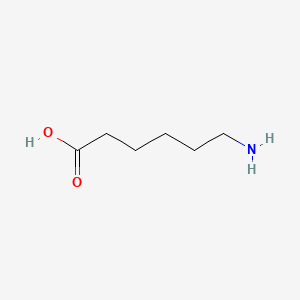 |
0.250 | ||
| ENC000250 | 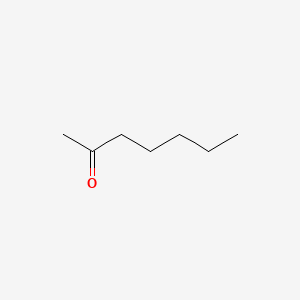 |
0.500 | D0O3AB | 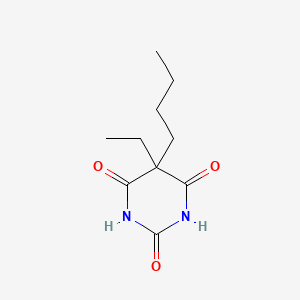 |
0.245 | ||
| ENC000245 | 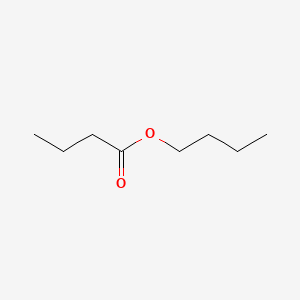 |
0.455 | D0OL6O | 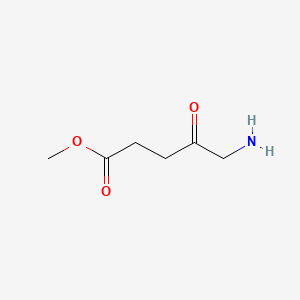 |
0.237 | ||
| ENC000235 |  |
0.452 | D03ZJE | 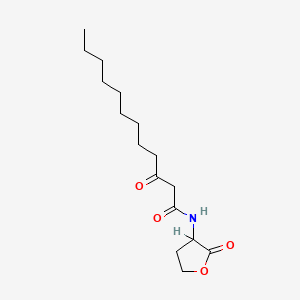 |
0.231 | ||
| ENC000254 | 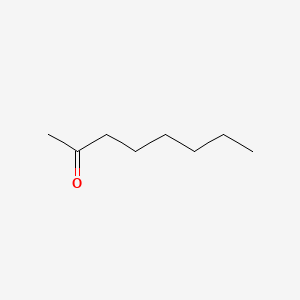 |
0.452 | D0Y4AW | 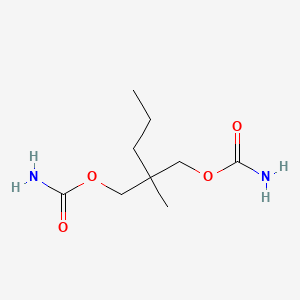 |
0.224 | ||
| ENC000315 | 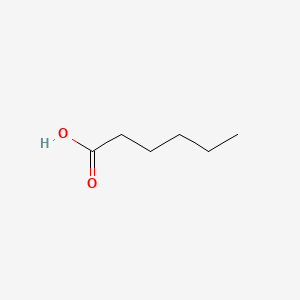 |
0.448 | D02HXS | 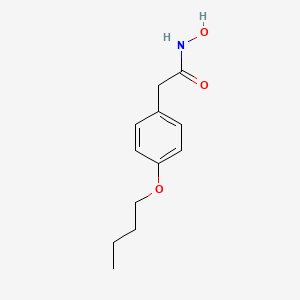 |
0.222 | ||